Low Amount of Salinomycin Greatly Increases Akt Activation, but Reduces Activated p70S6K Levels
Abstract
:1. Introduction
2. Results and Discussion
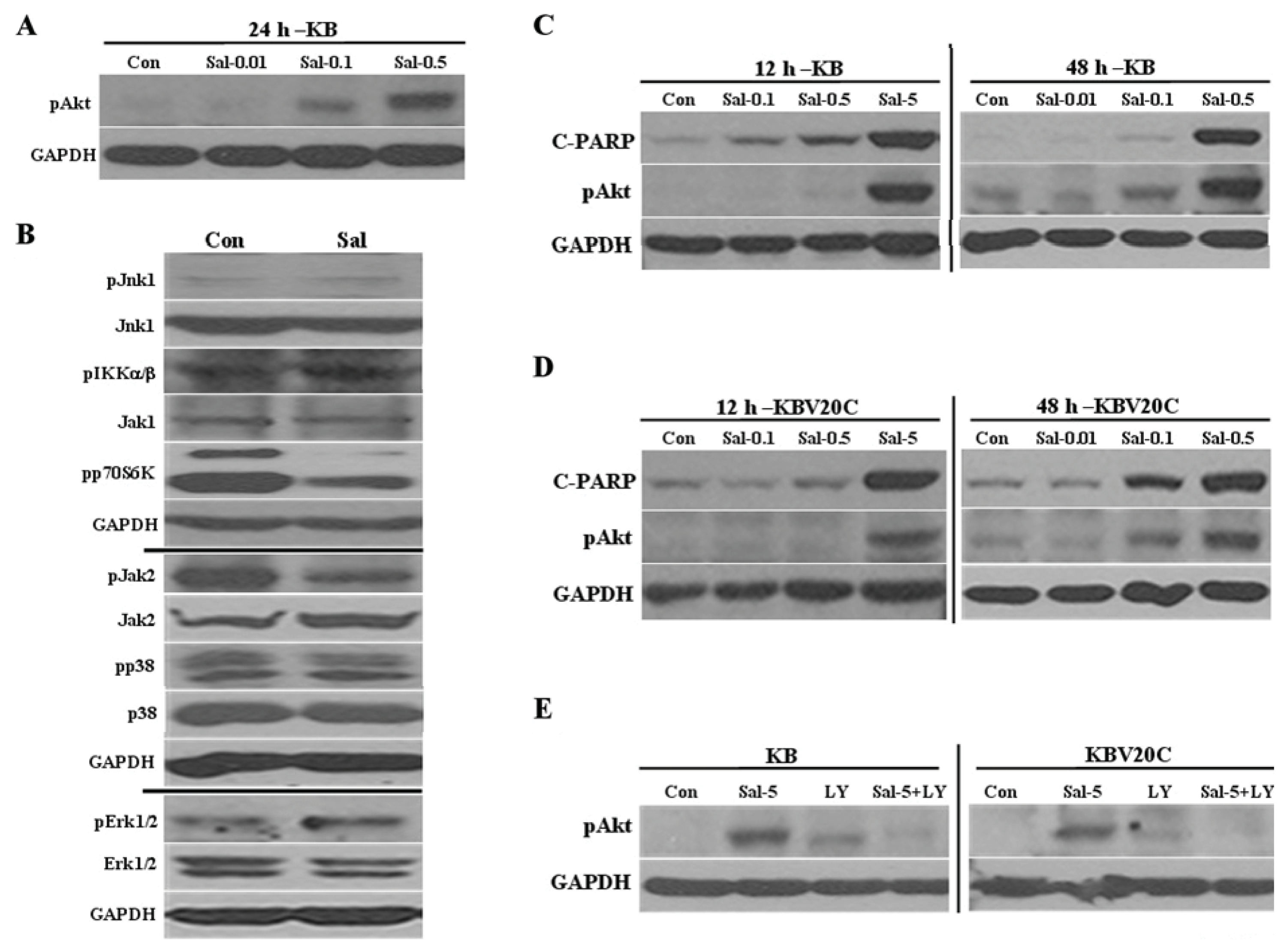
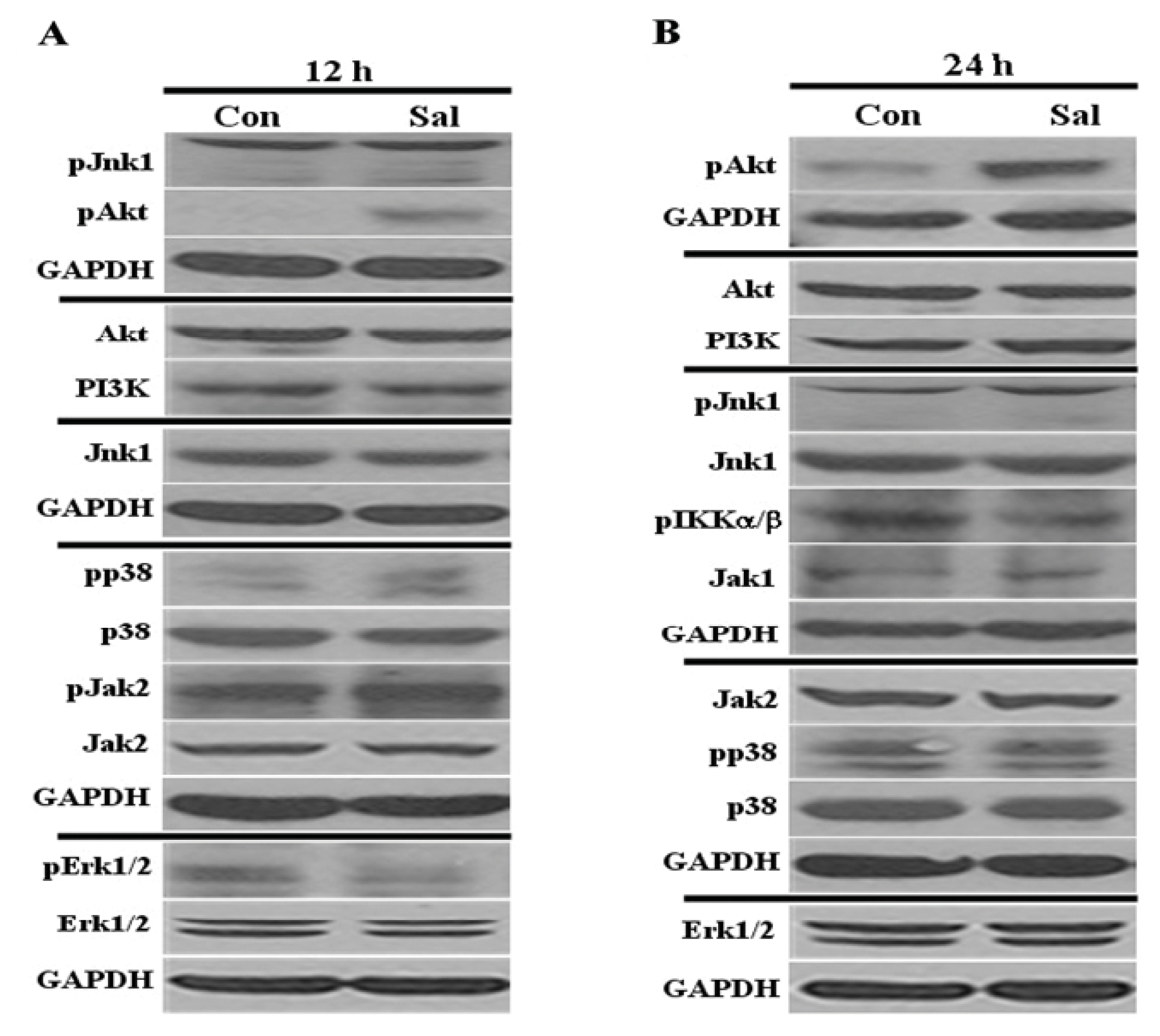
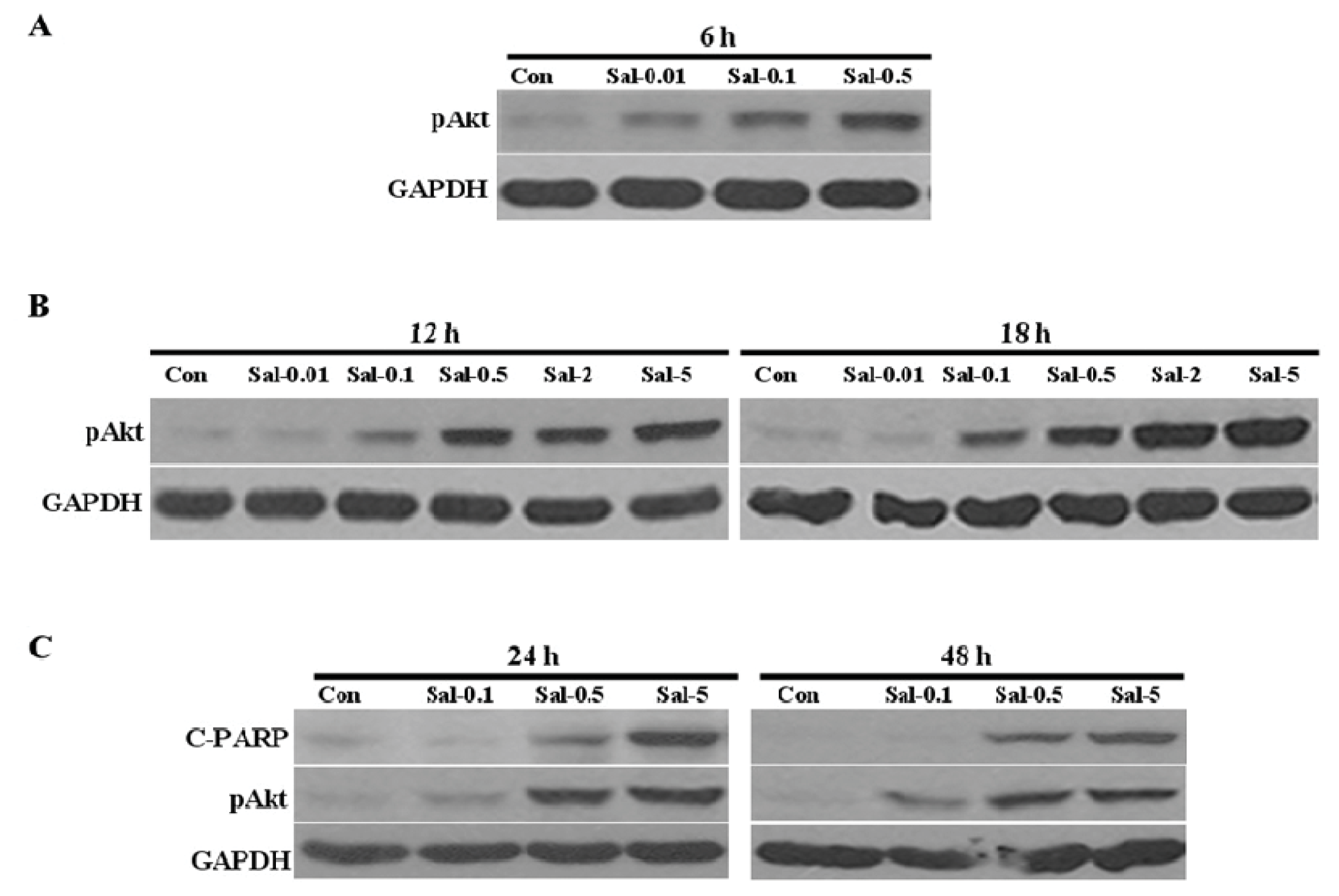
2.1. Low Concentration of Sal Highly Activates Akt
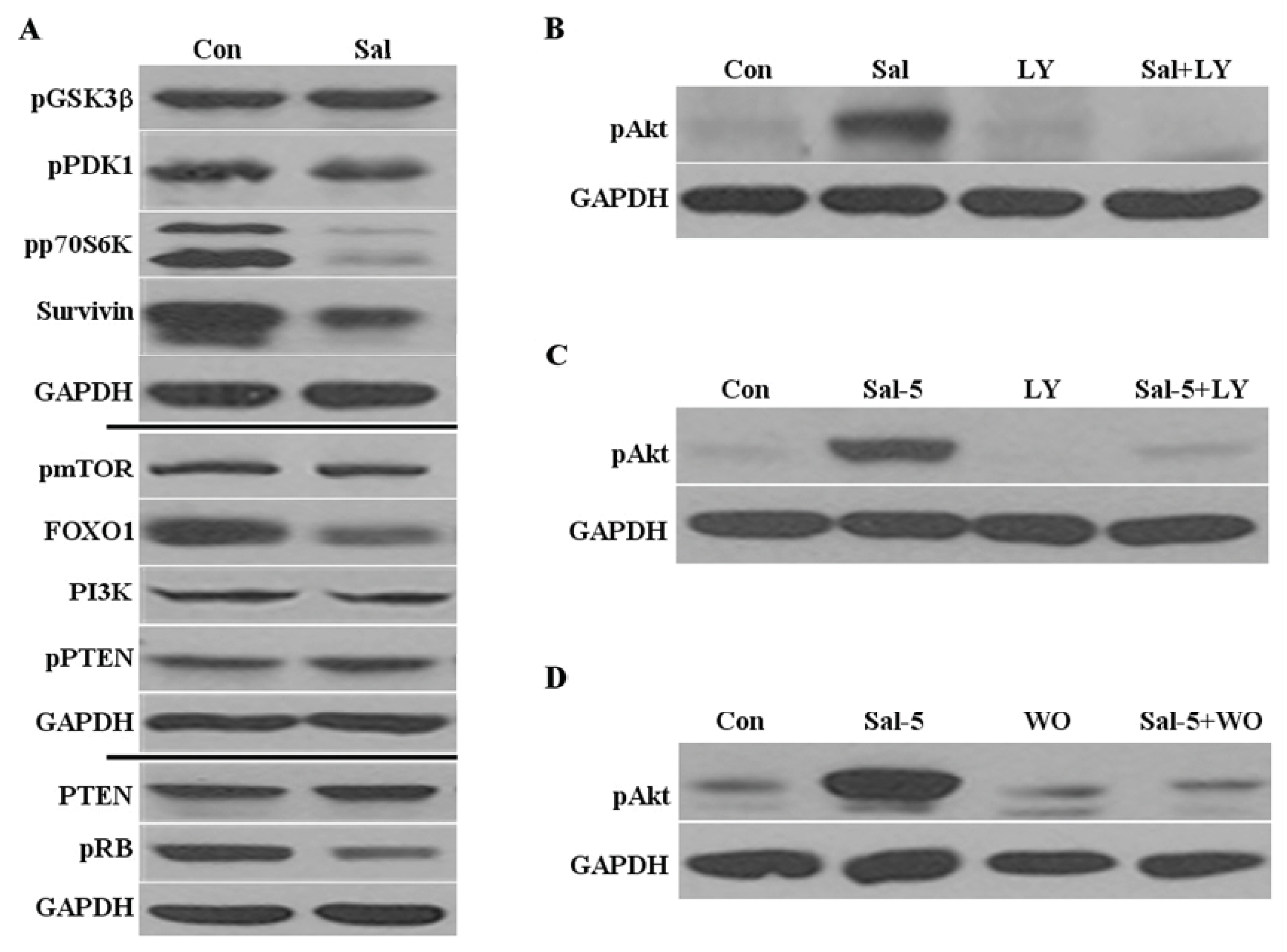
2.2. Sal Reduces Levels of Phosphorylated P70S6K, Survivin, and FOXO1
2.3. Akt Activation by Sal Requires the PI3K Pathway
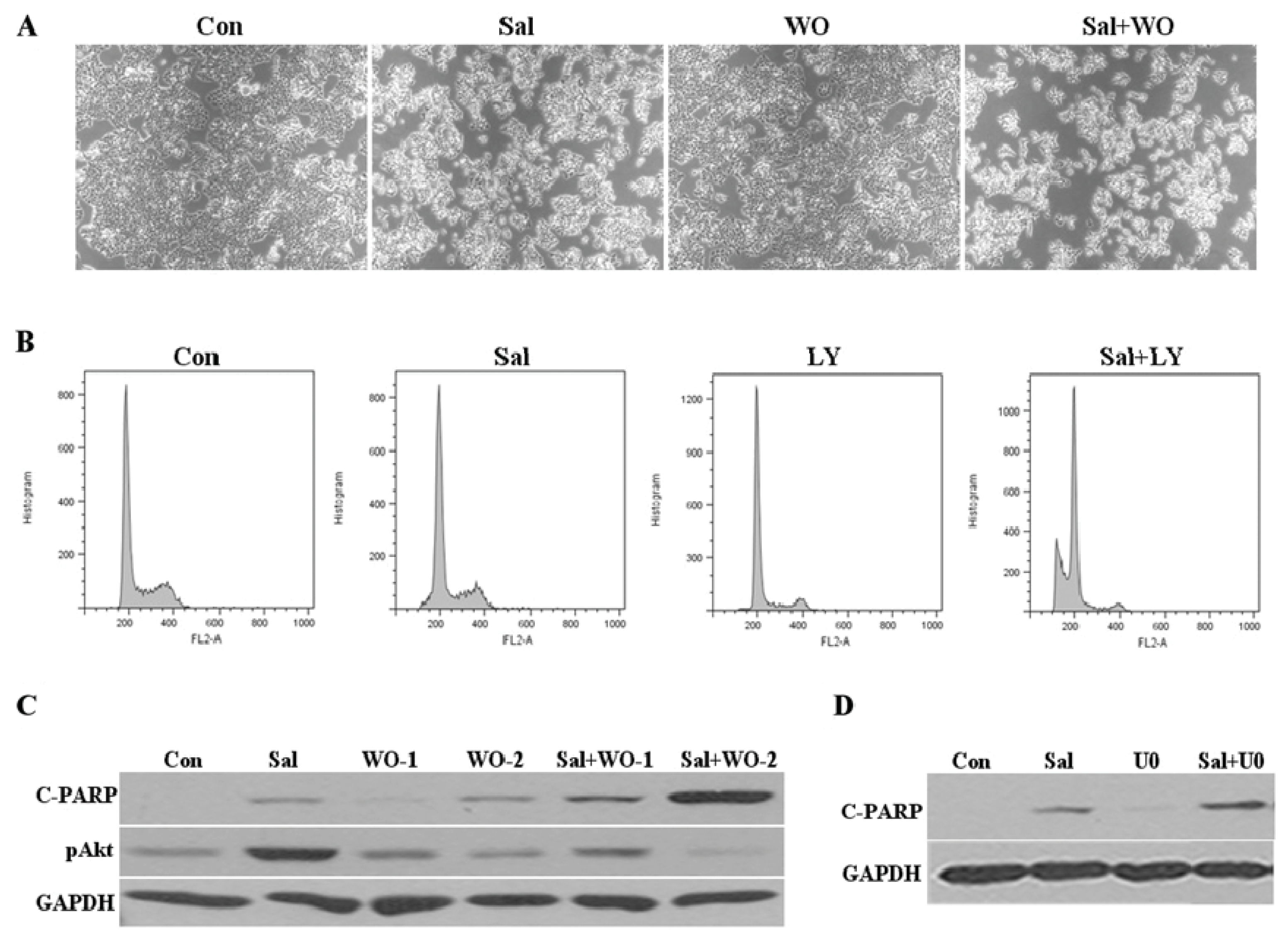
2.4. Akt Activation by Sal Correlates with Increased Cellular Apoptosis
2.5. Sal-Mediated Akt Activation Is Conserved in Other Cell Lines
2.6. Co-Treatment with an Akt Inhibitor Increases Apoptosis of Sal-Treated Cells
2.7. Discussion
3. Experimental Section
3.1. Reagents
3.2. Antibodies
3.3. Cell Culturing
3.4. Western Blot Analysis
3.5. Fluorescence-Activated Cell Sorting (FACS) Analysis
4. Conclusions
Acknowledgments
Appendix
Conflicts of Interest
References
- Miyazaki, Y.; Shibuya, M.; Sugawara, H.; Kawaguchi, O.; Hirsoe, C. Salinomycin, a new polyether antibiotic. J. Antibiot. (Tokyo) 1974, 27, 814–821. [Google Scholar]
- Mitani, M.; Yamanishi, T.; Miyazaki, Y. Salinomycin: A new monovalent cation ionophore. Biochem. Biophys. Res. Commun 1975, 66, 1231–1236. [Google Scholar]
- Mahmoudi, N.; de Julian-Ortiz, J.V.; Ciceron, L.; Galvez, J.; Mazier, D.; Danis, M.; Derouin, F.; Garcia-Domenech, R. Identification of new antimalarial drugs by linear discriminant analysis and topological virtual screening. J. Antimicrob. Chemother 2006, 57, 489–497. [Google Scholar]
- Bardsley, M.R.; Horvath, V.J.; Asuzu, D.T.; Lorincz, A.; Redelman, D.; Hayashi, Y.; Popko, L.N.; Young, D.L.; Lomberk, G.A.; Urrutia, R.A.; et al. Gastroenterology 2010, 139, 942–952.
- Dong, T.T.; Zhou, H.M.; Wang, L.L.; Feng, B.; Lv, B.; Zheng, M.H. Salinomycin selectively targets ‘CD133+’ cell subpopulations and decreases malignant traits in colorectal cancer lines. Ann. Surg. Oncol 2011, 18, 1797–1804. [Google Scholar]
- Fuchs, D.; Heinold, A.; Opelz, G.; Daniel, V.; Naujokat, C. Salinomycin induces apoptosis and overcomes apoptosis resistance in human cancer cells. Biochem. Biophys. Res. Commun 2009, 390, 743–749. [Google Scholar]
- Gong, C.; Yao, H.; Liu, Q.; Chen, J.; Shi, J.; Su, F.; Song, E. Markers of tumor-initiating cells predict chemoresistance in breast cancer. PLoS One 2010, 5, e15630. [Google Scholar]
- Gupta, P.B.; Onder, T.T.; Jiang, G.; Tao, K.; Kuperwasser, C.; Weinberg, R.A.; Lander, E.S. Identification of selective inhibitors of cancer stem cells by high-throughput screening. Cell 2009, 138, 645–659. [Google Scholar]
- Ketola, K.; Hilvo, M.; Hyotylainen, T.; Vuoristo, A.; Ruskeepaa, A.L.; Oresic, M.; Kallioniemi, O.; Iljin, K. Salinomycin inhibits prostate cancer growth and migration via induction of oxidative stress. Br. J. Cancer 2012, 106, 99–106. [Google Scholar]
- Lu, D.; Choi, M.Y.; Yu, J.; Castro, J.E.; Kipps, T.J.; Carson, D.A. Salinomycin inhibits Wnt signaling and selectively induces apoptosis in chronic lymphocytic leukemia cells. Proc. Natl. Acad. Sci. USA 2011, 108, 13253–13257. [Google Scholar]
- Tang, Q.L.; Zhao, Z.Q.; Li, J.C.; Liang, Y.; Yin, J.Q.; Zou, C.Y.; Xie, X.B.; Zeng, Y.X.; Shen, J.N.; Kang, T.; et al. Salinomycin inhibits osteosarcoma by targeting its tumor stem cells. Cancer Lett 2011, 311, 113–121. [Google Scholar]
- Zhang, G.N.; Liang, Y.; Zhou, L.J.; Chen, S.P.; Chen, G.; Zhang, T.P.; Kang, T.; Zhao, Y.P. Combination of salinomycin and gemcitabine eliminates pancreatic cancer cells. Cancer Lett 2011, 313, 137–144. [Google Scholar]
- Fuchs, D.; Daniel, V.; Sadeghi, M.; Opelz, G.; Naujokat, C. Salinomycin overcomes ABC transporter-mediated multidrug and apoptosis resistance in human leukemia stem cell-like KG-1a cells. Biochem. Biophys. Res. Commun 2010, 394, 1098–1104. [Google Scholar]
- Kim, W.K.; Kim, J.H.; Yoon, K.; Kim, S.; Ro, J.; Kang, H.S.; Yoon, S. Salinomycin, a p-glycoprotein inhibitor, sensitizes radiation-treated cancer cells by increasing DNA damage and inducing G2 arrest. Invest. New Drugs 2012, 30, 1311–1318. [Google Scholar]
- Riccioni, R.; Dupuis, M.L.; Bernabei, M.; Petrucci, E.; Pasquini, L.; Mariani, G.; Cianfriglia, M.; Testa, U. The cancer stem cell selective inhibitor salinomycin is a p-glycoprotein inhibitor. Blood Cells Mol. Dis 2010, 45, 86–92. [Google Scholar]
- Kim, J.H.; Chae, M.; Kim, W.K.; Kim, Y.J.; Kang, H.S.; Kim, H.S.; Yoon, S. Salinomycin sensitizes cancer cells to the effects of doxorubicin and etoposide treatment by increasing DNA damage and reducing p21 protein. Br. J. Pharmacol 2011, 162, 773–784. [Google Scholar]
- Kim, J.H.; Yoo, H.I.; Kang, H.S.; Ro, J.; Yoon, S. Salinomycin sensitizes antimitotic drugs-treated cancer cells by increasing apoptosis via the prevention of G2 arrest. Biochem. Biophys. Res. Commun 2012, 418, 98–103. [Google Scholar]
- Sebolt-Leopold, J.S. Development of anticancer drugs targeting the MAP kinase pathway. Oncogene 2000, 19, 6594–6599. [Google Scholar]
- Pommier, Y.; Sordet, O.; Antony, S.; Hayward, R.L.; Kohn, K.W. Apoptosis defects and chemotherapy resistance: Molecular interaction maps and networks. Oncogene 2004, 23, 2934–2949. [Google Scholar]
- Ohtsuka, T.; Buchsbaum, D.; Oliver, P.; Makhija, S.; Kimberly, R.; Zhou, T. Synergistic induction of tumor cell apoptosis by death receptor antibody and chemotherapy agent through JNK/p38 and mitochondrial death pathway. Oncogene 2003, 22, 2034–2044. [Google Scholar]
- Brozovic, A.; Osmak, M. Activation of mitogen-activated protein kinases by cisplatin and their role in cisplatin-resistance. Cancer Lett 2007, 251, 1–16. [Google Scholar]
- Arslan, M.A.; Kutuk, O.; Basaga, H. Protein kinases as drug targets in cancer. Curr. Cancer Drug Targets 2006, 6, 623–634. [Google Scholar]
- Bradham, C.; McClay, D.R. P38 MAPK in development and cancer. Cell Cycle 2006, 5, 824–828. [Google Scholar]
- Knuefermann, C.; Lu, Y.; Liu, B.; Jin, W.; Liang, K.; Wu, L.; Schmidt, M.; Mills, G.B.; Mendelsohn, J.; Fan, Z. HER2/PI-3K/Akt activation leads to a multidrug resistance in human breast adenocarcinoma cells. Oncogene 2003, 22, 3205–3212. [Google Scholar]
- Kohno, M.; Pouyssegur, J. Targeting the ERK signaling pathway in cancer therapy. Ann. Med 2006, 38, 200–211. [Google Scholar]
- Kim, J.H.; Kim, T.Y.; Kim, H.S.; Hong, S.; Yoon, S. Lower salinomycin concentration increases apoptotic detachment in high-density cancer cells. Int. J. Mol. Sci 2012, 13, 13169–13182. [Google Scholar]
- Chen, Q.; Ganapathy, S.; Singh, K. P.; Shankar, S.; Srivastava, R.K. Resveratrol induces growth arrest and apoptosis through activation of FOXO transcription factors in prostate cancer cells. PLoS One 2010, 5, e15288. [Google Scholar]
- Kim, J.H.; Kim, T.H.; Kang, H.S.; Ro, J.; Kim, H.S.; Yoon, S. SP600125, an inhibitor of Jnk pathway, reduces viability of relatively resistant cancer cells to doxorubicin. Biochem. Biophys. Res. Commun 2009, 387, 450–455. [Google Scholar]
- Kim, N.H.; Kim, S.N.; Oh, J.S.; Lee, S.; Kim, Y.K. Anti-mitotic potential of 7-diethylamino-3(2′-benzoxazolyl)-coumarin in 5-fluorouracil-resistant human gastric cancer cell line SNU620/5-FU. Biochem. Biophys. Res. Commun 2012, 418, 616–621. [Google Scholar]
- Bennett, B.L.; Sasaki, D.T.; Murray, B.W.; O’Leary, E.C.; Sakata, S.T.; Xu, W.; Leisten, J.C.; Motiwala, A.; Pierce, S.; Satoh, Y.; et al. SP600125, an anthrapyrazolone inhibitor of Jun N-terminal kinase. Proc. Natl. Acad. Sci. USA 2001, 98, 13681–13686. [Google Scholar]
- Das, R.; Mahabeleshwar, G.H.; Kundu, G.C. Osteopontin induces AP-1-mediated secretion of urokinase-type plasminogen activator through c-Src-dependent epidermal growth factor receptor transactivation in breast cancer cells. J. Biol. Chem 2004, 279, 11051–11064. [Google Scholar]
- Liu, Y.; Mueller, B.M. Protease-activated receptor-2 regulates vascular endothelial growth factor expression in MDA-MB-231 cells via MAPK pathways. Biochem. Biophys. Res. Commun 2006, 344, 1263–12670. [Google Scholar]
- Lee, K.H.; Moon, K.J.; Kim, H.S.; Yoo, B.C.; Park, S.; Lee, H.; Kwon, S.; Lee, E.S.; Yoon, S. Increased cytoplasmic levels of CIS, SOCS1, SOCS2, or SOCS3 are required for nuclear translocation. FEBS Lett 2008, 582, 2319–23124. [Google Scholar]
- Kim, J.H.; Lee, S.; Ro, J.; Kang, H.S.; Kim, H.S.; Yoon, S. Jnk signaling pathway-mediated regulation of Stat3 activation is linked to the development of doxorubicin resistance in cancer cell lines. Biochem. Pharmacol. 2010, 79, 373–380. [Google Scholar]

© 2013 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Kim, J.-H.; Choi, A.-R.; Kim, Y.K.; Kim, H.S.; Yoon, S. Low Amount of Salinomycin Greatly Increases Akt Activation, but Reduces Activated p70S6K Levels. Int. J. Mol. Sci. 2013, 14, 17304-17318. https://doi.org/10.3390/ijms140917304
Kim J-H, Choi A-R, Kim YK, Kim HS, Yoon S. Low Amount of Salinomycin Greatly Increases Akt Activation, but Reduces Activated p70S6K Levels. International Journal of Molecular Sciences. 2013; 14(9):17304-17318. https://doi.org/10.3390/ijms140917304
Chicago/Turabian StyleKim, Ju-Hwa, Ae-Ran Choi, Yong Kee Kim, Hyung Sik Kim, and Sungpil Yoon. 2013. "Low Amount of Salinomycin Greatly Increases Akt Activation, but Reduces Activated p70S6K Levels" International Journal of Molecular Sciences 14, no. 9: 17304-17318. https://doi.org/10.3390/ijms140917304



