Isolation and Structure Characterization of an Antioxidative Glycopeptide from Mycelial Culture Broth of a Medicinal Fungus
Abstract
:1. Introduction
2. Results and Discussion
2.1. Isolation and Molecular Profiles of Cs-GP1 from EPS-2

| Fraction | Protein Content (%) | Mw (kDa) | Mole Ratio | ||||
|---|---|---|---|---|---|---|---|
| Man | Glc | GlcA | Gal | GalN | |||
| OF-IV | 30.1 | 13 | -- | 0.1 | -- | -- | 2.1 |
| OF-V | 50.5 | 6.0 | 1.0 | 3.2 | 1.5 | 1.0 | 0.96 |
| Cs-GP1 | 52.5 | 6.0 | 1.0 | 3.2 | -- | 0.2 | 0.3 |
2.2. Sugar and Amino Acid Constituents of Cs-GP1

| Amino Acid | Content (μg/mg) * | Mole Ratio ** | MW | |
|---|---|---|---|---|
| 1 | Asp | 66.89 ± 1.23 | 3.28 | 133 |
| 2 | Thr | 9.76 ± 0.31 | 0.54 | 119 |
| 3 | Ser | 33.56 ± 1.11 | 2.09 | 105 |
| 4 | Glu | 76.61 ± 2.41 | 3.40 | 147 |
| 5 | Gly | 40.61 ± 1.48 | 3.53 | 75 |
| 6 | Ala | 26.27 ± 0.93 | 1.93 | 89 |
| 7 | Cys | 40.61 ± 1.56 | 1.10 | 240 |
| 8 | Val | 7.86 ± 0.36 | 0.44 | 149 |
| 9 | Ile | 5.91 ± 0.44 | 0.29 | 131 |
| 10 | Leu | 7.12 ± 0.28 | 0.35 | 131 |
| 11 | Tyr | 3.96 ± 0.19 | 0.14 | 181 |
| 12 | Phe | 30.52 ± 1.13 | 1.20 | 165 |
| 13 | Lys | 30.22 ± 0.79 | 1.35 | 146 |
| 14 | His | 11.42 ± 0.52 | 0.48 | 155 |
| 15 | Arg | 26.61 ± 0.72 | 1.00 | 174 |
| 16 | Pro | 19.59 ± 0.67 | 1.11 | 115 |
| Total amino acid | 437.52 ± 16.15 | |||
2.3. IR and NMR Spectra
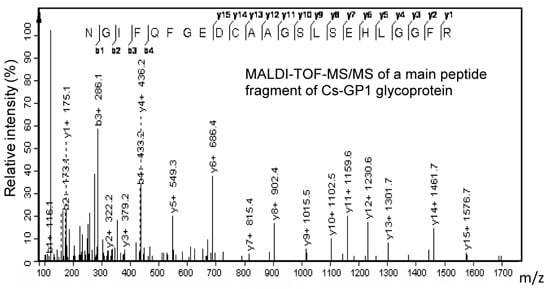
2.4. Amino Acid Sequence of Peptide Chain

| Fragments | Mass | Sequence |
|---|---|---|
| A | 1758 | DCAAGSLSEHLGGFRE * |
| B | 1412 | AAGSLSEHLGGFR |
| C | 1359 | AGSLSEHLGGFR |
| D | 2470 | NGIFQFGEDCAAGSLSEHLGGFR |
| E | 1930 | KNGIFQFGEDCAAGSLSE |
| F | 1987 | GKNGIFQFGEDCAAGSLSE |
| G | 2172 | HLGGFREFLKAGNLE |
| Whole chain | 4102 | GKNGIFQFGEDCAAGSLSEHLGGFREFREFLKAGNLE |
2.5. Antioxidant Activities

2.6. Discussion
3. Experimental Section
3.1. Materials
3.2. Isolation and Purification of EPS from Cs-HK1 Mycelial Culture

3.3. Analysis of Cs-GP1 Molecular Composition and Properties
3.3.1. Monosaccharide, Amino Acid and Protein Contents
3.3.2. Average Molecular Weight
3.3.3. NMR and IR Spectroscopy
3.4. Analysis of Peptide Chain Sequence
3.4.1. In-Gel Digestion
3.4.2. Mass Spectrometry
3.5. Antioxidant Activity Assays
4. Conclusions
Supplementary Materials
Supplementary Files
Supplementary File 1Acknowledgments
Author Contributions
Conflicts of Interest
References
- Ooi, V.E.; Liu, F. Immunomodulation and anti-cancer activity of polysaccharide-protein complexes. Curr. Med. Chem. 2000, 7, 715–729. [Google Scholar]
- Xu, X.; Yan, H.; Chen, J.; Zhang, X. Bioactive proteins from mushrooms. Biotechnol. Adv. 2011, 29, 667–674. [Google Scholar]
- Samaranayaka, A.G.; Li-Chan, E.C. Food-derived peptidic antioxidants: A review of their production, assessment, and potential applications. J. Funct. Foods 2011, 3, 229–254. [Google Scholar]
- Li, S.P.; Zhao, K.J.; Ji, Z.N.; Song, Z.H.; Dong, T.T.X.; Lo, C.K.; Cheung, J.K.H.; Zhu, S.Q.; Tsim, K.W.K. A polysaccharide isolated from Cordyceps sinensis, a traditional Chinese medicine, protects PC12 cells against hydrogen peroxide-induced injury. Life Sci. 2003, 73, 2503–2513. [Google Scholar]
- Zhu, J.S.; Halpern, G.M.; Jones, K. The scientific rediscovery of a precious ancient Chinese herbal regimen: Cordyceps sinensis—Part II. J. Altern. Complement. Med. 1998, 4, 429–457. [Google Scholar]
- Paterson, R.R.M. Cordyceps: A traditional Chinese medicine and another fungal therapeutic biofactory? Phytochemistry 2008, 7, 1469–1495. [Google Scholar] [CrossRef] [Green Version]
- Wong, J.H.; Ng, T.B.; Wang, H.; Sze, S.C.W.; Zhang, K.Y.; Li, Q.; Lu, X. Cordymin, an antifungal peptide from the medicinal fungus Cordyceps militaris. Phytomedicine 2011, 18, 387–392. [Google Scholar]
- Wang, J.; Liu, Y.-M.; Cao, W.; Yao, K.-W.; Liu, Z.-Q.; Guo, J.-Y. Anti-inflammation and antioxidant effect of Cordymin, a peptide purified from the medicinal mushroom Cordyceps sinensis, in middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Met. Brain Dis. 2012, 27, 159–165. [Google Scholar]
- Leung, P.H.; Zhang, Q.X.; Wu, J.Y. Mycelium cultivation, chemical composition and antitumour activity of a Tolypocladium sp. fungus isolated from wild Cordyceps sinensis. J. Appl. Microbiol. 2006, 101, 275–283. [Google Scholar]
- Leung, P.H.; Zhao, S.; Ho, K.P.; Wu, J.Y. Chemical properties and antioxidant activity of exopolysaccharides from mycelial culture of Cordyceps sinensis fungus Cs-HK1. Food Chem. 2009, 114, 1251–1256. [Google Scholar]
- Huang, Q.L.; Siu, K.C.; Wang, W.Q.; Cheung, Y.C.; Wu, J.Y.; Huang, Q.-L.; Siu, K.-C.; Wang, W.-Q.; Cheung, Y.-C.; Wu, J.-Y. Fractionation, characterization and antioxidant activity of exopolysaccharides from fermentation broth of a Cordyceps sinensis fungus. Process Biochem. 2013, 48, 380–386. [Google Scholar]
- Chen, S.; Siu, K.-C.; Wang, W.-Q.; Liu, X.-X.; Wu, J.-Y. Structure and antioxidant activity of a novel poly-N-acetylhexosamine produced by a medicinal fungus. Carbohydr. Polym. 2013, 3, 332–338. [Google Scholar]
- Fabian, H.; Mäntele, W. Infrared spectroscopy of proteins. In Handbook of Vibrational Spectroscopy; John Wiley & Sons, Ltd.: Chichester, NJ, USA, 2002; Volume 5, pp. 3399–3425. [Google Scholar]
- Barth, A. Infrared spectroscopy of proteins. Biochim. Biophys. Acta BBA-Bioenerg. 2007, 1767, 1073–1101. [Google Scholar]
- Chen, S.; Xu, J.; Xue, C.; Dong, P.; Sheng, W.; Yu, G.; Chai, W. Sequence determination of a non-sulfated glycosaminoglycan-like polysaccharide from melanin-free ink of the squid Ommastrephes bartrami by negative-ion electrospray tandem mass spectrometry and NMR spectroscopy. Glycoconj. J. 2008, 25, 481–492. [Google Scholar]
- Chen, S.; Li, G.; Wu, N.; Guo, X.; Liao, N.; Ye, X.; Liu, D.; Xue, C.; Chai, W. Sulfation pattern of the fucose branch is important for the anticoagulant and antithrombotic activities of fucosylated chondroitin sulfates. Biochim. Biophys. Acta BBA-Gen. Subj. 2013, 1830, 3054–3066. [Google Scholar]
- Helenius, A.; Aebi, M. Intracellular functions of N-linked glycans. Science 2001, 291, 2364–2369. [Google Scholar]
- Petrescu, A.J.; Wormald, M.R.; Dwek, R.A. Structural aspects of glycomes with a focus on N-glycosylation and glycoprotein folding. Curr. Opin. Struct. Biol. 2006, 16, 600–607. [Google Scholar]
- Varki, A. Biological roles of oligosaccharides: All of the theories are correct. Glycobiology 1993, 3, 97–130. [Google Scholar]
- Dwek, R.A. Glycobiology: Toward understanding the function of sugars. Chem. Rev. 1996, 96, 683–720. [Google Scholar]
- Dwek, R.A.; Butters, T.D.; Platt, F.M.; Zitzmann, N. Targeting glycosylation as a therapeutic approach. Nat. Rev. Drug Discov. 2002, 1, 65–75. [Google Scholar]
- Haltiwanger, R.S.; Lowe, J.B. Role of glycosylation in development. Annu. Rev. Biochem. 2004, 73, 491–537. [Google Scholar]
- Dube, D.H.; Bertozzi, C.R. Glycans in cancer and inflammation—Potential for therapeutics and diagnostics. Nat. Rev. Drug Discov. 2005, 4, 477–488. [Google Scholar]
- Chen, H.M.; Muramoto, K.; Yamauchi, F. Structural analysis of antioxidative peptides from soybean β-conglycinin. J. Agric. Food Chem. 1995, 43, 574–578. [Google Scholar]
- Mendis, E.; Rajapakse, N.; Byun, H.G.; Kim, S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar]
- Lowry, O.H.; Rosebrough, N.J.; Farr, A.L.; Randall, R.J. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951, 193, 265–275. [Google Scholar]
- Shevchenko, A.; Tomas, H.; Havlis, J.; Olsen, J.V.; Mann, M. In-gel digestion for mass spectrometric characterization of proteins and proteomes. Nat. Protoc. 2007, 1, 2856–2860. [Google Scholar]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of antioxidant power by the FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, J.-Y.; Chen, X.; Siu, K.-C. Isolation and Structure Characterization of an Antioxidative Glycopeptide from Mycelial Culture Broth of a Medicinal Fungus. Int. J. Mol. Sci. 2014, 15, 17318-17332. https://doi.org/10.3390/ijms151017318
Wu J-Y, Chen X, Siu K-C. Isolation and Structure Characterization of an Antioxidative Glycopeptide from Mycelial Culture Broth of a Medicinal Fungus. International Journal of Molecular Sciences. 2014; 15(10):17318-17332. https://doi.org/10.3390/ijms151017318
Chicago/Turabian StyleWu, Jian-Yong, Xia Chen, and Ka-Chai Siu. 2014. "Isolation and Structure Characterization of an Antioxidative Glycopeptide from Mycelial Culture Broth of a Medicinal Fungus" International Journal of Molecular Sciences 15, no. 10: 17318-17332. https://doi.org/10.3390/ijms151017318
APA StyleWu, J.-Y., Chen, X., & Siu, K.-C. (2014). Isolation and Structure Characterization of an Antioxidative Glycopeptide from Mycelial Culture Broth of a Medicinal Fungus. International Journal of Molecular Sciences, 15(10), 17318-17332. https://doi.org/10.3390/ijms151017318