Boric Ester-Type Molten Salt via Dehydrocoupling Reaction
Abstract
:1. Introduction

2. Results and Discussion

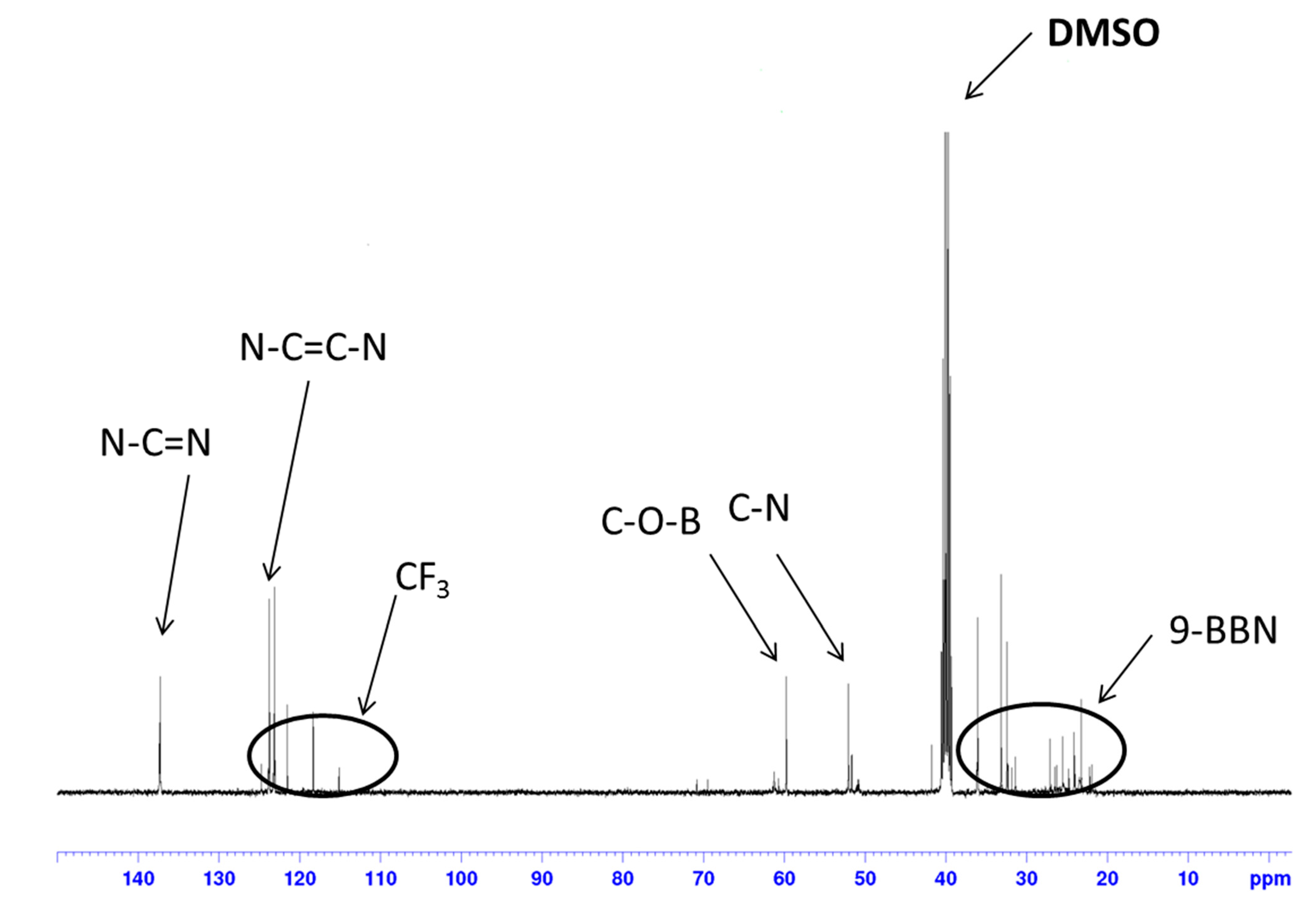




3. Experimental Section
3.1. Instruments and Materials

| Sample | A (S cm−1 K1/2) | B (K) | T0 (K) | R2 |
|---|---|---|---|---|
 | 5.27 | 985 | 200 | 0.996 |
 | 0.954 | 1,013 | 200 | 0.998 |
 | 0.302 | 796 | 200 | 0.997 |
 | 1.47 | 907 | 200 | 0.999 |
 | 1.54 | 879 | 200 | 0.999 |
3.2. Synthesis of Organoboron Molten Salt
3.2.1. Ion Exchange Reaction of 1-(2-Hydroxyethyl)-3-methylimidazolium Chloride
3.2.2. Dehydrocoupling Reaction of 1-(2-Hydroxyethyl)-3-methylimidazolium NTf2
4. Conclusions
Author Contributioins
Conflicts of Interest
References
- Armand, M.; Tarascon, J.M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar]
- Scrosati, B.; Garche, J. Lithium batteries: Status, prospects and future. J. Power Sources 2010, 195, 2419–2430. [Google Scholar]
- Ohno, H. Electrochemical Aspects of Ionic Liquids; Wiley Interscience: Hoboken, NJ: USA, 2005. [Google Scholar]
- Ohno, H. Functional design of ionic liquid. Bull. Chem. Soc. Jpn. 2006, 79, 1665–1680. [Google Scholar]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Pringle, J.M.; Sun, J.; Annat, G.; Neil, W.; Izgorodina, E.I. Ionic liquids in electrochemical devices and processes: Managing interfacial chemistry. Acc. Chem. Res. 2007, 40, 1165–1173. [Google Scholar]
- Matsumi, N.; Miyake, M.; Ohno, H. Molten salts bearing anion receptor. Chem. Commun. 1994, 2852–2853. [Google Scholar]
- Matsumi, N.; Sugai, K.; Miyake, M.; Ohno, H. Polymerized ionic liquids via hydroboration polymerization as single ion conductive polymer electrolytes. Macromolecules 2006, 39, 6924–6927. [Google Scholar]
- Yoshizawa, M.; Hirao, M.; Ito-Akita, K.; Ohno, H. Ion conduction in zwitterionic-type molten salts and their polymers. J. Mater. Chem. 2001, 11, 1057–1062. [Google Scholar]
- Yoshizawa, M.; Narita, A.; Ohno, H. Design of ionic liquids for electrochemical applications. Aust. J. Chem. 2004, 57, 139–144. [Google Scholar]
- Narita, A.; Shibayama, W.; Sakamoto, K.; Mizumo, T.; Matsumi, N.; Ohno, H. Lithium ion conduction in an organoborate zwitterion-LiTFSI mixture. Chem. Commun. 2006, 1926–1928. [Google Scholar]
- Mehta, M.A.; Fujinami, T. Li+ transference number enhancement in polymer electrolytes by incorporation of anion trapping boroxine rings into the polymer host. Chem. Lett. 1997, 9, 915–916. [Google Scholar]
- Hirakimoto, T.; Nishiura, M.; Watanabe, M. Effects of addition of a boric acid ester monomer to electrolyte solutions and gel electrolytes on their ionic transport properties. Electrochim. Acta 2001, 46, 1609–1614. [Google Scholar]
- Matsumi, N.; Sugai, K.; Ohno, H. Ion conductive characteristics of alkylborane type and boric ester type polymer electrolytes derived from mesitylborane. Macromolecules 2003, 36, 2321–2326. [Google Scholar]
- Matsumi, N.; Sugai, K.; Ohno, H. Selective ion transport in organoboron polymer electrolytes bearing a mesitylborane unit. Macromolecules 2002, 35, 5731–5733. [Google Scholar]
- Matsumi, N.; Mizumo, T.; Ohno, H. Single ion conductive characteristics of poly(organoboron halide)-imidazole complex. Polym. Bull. 2004, 51, 389–394. [Google Scholar]
- Matsumi, N.; Sugai, K.; Sakamoto, K.; Mizumo, T.; Ohno, H. Direct synthesis of poly(lithium organoborate)s and their ion conductive properties. Macromolecules 2005, 38, 4951–4954. [Google Scholar]
- Matsumi, N.; Nakashiba, M.; Mizumo, T.; Ohno, H. Novel polymer/salt hybrids composed of comb like organoboron polymer electrolyte and boron stabilized imido anion. Macromolecules 2005, 38, 2040–2042. [Google Scholar]
- Matsumi, N.; Kagata, A.; Aoi, K. Synthesis of supramolecular solid polymer electrolytes via self-assembly of diborylated ionic liquid. J. Power Sources 2010, 195, 6182–6186. [Google Scholar]
- Matsumi, N.; Yoshioka, N.; Aoi, K. Synthesis of boric ester type ion-gels by dehydrocoupling of cellulose with hydroboranes in ionic liquid. Solid State Ionics 2012, 226, 37–40. [Google Scholar]
- Matsumi, N.; Nakamura, Y.; Aoi, K.; Watanabe, T.; Mizumo, T.; Ohno, H. Enhanced ionic conduction in organoboron ion gels facilely designed via condensation of cellulose with boric acids in ionic liquids. Polym. J. 2009, 41, 437–441. [Google Scholar]
- Mizumo, T.; Watanabe, T.; Matsumi, N.; Ohno, H. Preparation of ion conductive inorganic-organic composite systems by in situ sol-gel reaction of polymerizable ionic liquids. Polym. Adv. Technol. 2008, 19, 1445–1450. [Google Scholar]
- Hardy, L.C.; Shriver, D.F. Preparation and electrical response of solid polymer electrolytes with only one mobile species. J. Am. Chem. Soc. 1985, 107, 3823–3828. [Google Scholar]
- Tsuchida, E.; Kobayashi, N.; Ohno, H. Single ion conduction in poly(oligo(oxyethylene)methacrylate)-co-(alkali-metalmethacrylates). Macromolecules 1988, 21, 96–100. [Google Scholar]
- Vogel, H. The temperature dependence law of the viscosity of fluids. Phys. Z. 1921, 22, 645–646. [Google Scholar]
- Fulcher, G.S. Analysis of recent measurements of the viscosity of glasses. J. Am. Ceram. Soc. 1925, 8, 339. [Google Scholar]
- Tamman, G.; Hesse, W. Die abhangigkeit der viskositat von der temperature bei unterkuhltenflussigkeiten. Z. Anorg. Allg. Chem. 1926, 156, 245–257. (In German) [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsumi, N.; Toyota, Y.; Joshi, P.; Puneet, P.; Vedarajan, R.; Takekawa, T. Boric Ester-Type Molten Salt via Dehydrocoupling Reaction. Int. J. Mol. Sci. 2014, 15, 21080-21089. https://doi.org/10.3390/ijms151121080
Matsumi N, Toyota Y, Joshi P, Puneet P, Vedarajan R, Takekawa T. Boric Ester-Type Molten Salt via Dehydrocoupling Reaction. International Journal of Molecular Sciences. 2014; 15(11):21080-21089. https://doi.org/10.3390/ijms151121080
Chicago/Turabian StyleMatsumi, Noriyoshi, Yoshiyuki Toyota, Prerna Joshi, Puhup Puneet, Raman Vedarajan, and Toshihiro Takekawa. 2014. "Boric Ester-Type Molten Salt via Dehydrocoupling Reaction" International Journal of Molecular Sciences 15, no. 11: 21080-21089. https://doi.org/10.3390/ijms151121080




