PLGA Biodegradable Nanoparticles Containing Perphenazine or Chlorpromazine Hydrochloride: Effect of Formulation and Release
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preparation and Characterization of Poly(dl-lactide-co-glycolide) (PLGA) Nanoparticles (NPs)
| Name of Compound | Structural Formula | MW (g∙mol−1) |
|---|---|---|
| Perphenazine (PPH) | 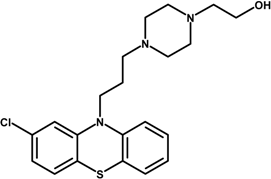 | 403.97 |
| Chlorpromazine hydrochloride (CPZ-HCl) | 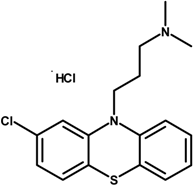 | 355.30 |
| Poly(dl-lactide-co-glycolide) (PLGA: 50:50) |  | 54,000 69,000 |
2.2. Size Distribution and Polydispersity
| PLGA (%) | TDL (%) | PVA (%) | Power Son. (%) | PPH | CPZ-HCl | ||
|---|---|---|---|---|---|---|---|
| NPs (nm) | PDI | NPs Size (nm) | PDI | ||||
| 0.8 | 20 | 0.5 | 30 | 340.5 ± 17.8 | 0.112 ± 0.016 | 374.3 ± 10.1 | 0.149 ± 0.003 |
| 1.3 | 20 | 0.5 | 30 | 382.5 ± 36.5 | 0.119 ± 0.022 | 374.3 ± 10.1 | 0.149 ± 0.003 |
| 1.6 | 20 | 0.5 | 30 | 376.6 ± 28.6 | 0.107 ± 0.034 | 426.0 ± 19.4 | 0.123 ± 0.037 |
| 1.3 | 10 | 0.5 | 30 | 367.2 ± 22.7 | 0.138 ± 0.013 | 382.4 ± 10.2 | 0.178 ± 0.030 |
| 1.3 | 30 | 0.5 | 30 | 397.1 ± 31.1 | 0.149 ± 0.048 | 397.9 ± 28.8 | 0.098 ± 0.016 |
| 1.3 | 20 | 0.3 | 30 | – | – | 476.7 ± 11.2 | 0.243 ± 0.032 |
| 1.3 | 20 | 0.7 | 30 | 325.5 ± 32.4 | 0.105 ± 0.020 | 395.2 ± 24.4 | 0.150 ± 0.016 |
| 1.3 | 20 | 0.5 | 50 | 363.0 ± 30.9 | 0.109 ± 0.010 | 403.5 ± 12.2 | 0.148 ± 0.013 |
| 1.3 | 20 | 0.5 | 70 | 419.1 ± 23.4 | 0.158 ± 0.004 | 443.0 ± 11.1 | 0.183 ± 0.030 |
2.3. Drug Efficiency and Drug Loading
| PLGA (%) | TDL (%) | PVA (%) | Power Son. (%) | PPH | CPZ-HCl | ||
|---|---|---|---|---|---|---|---|
| EE (%) | DL (%) | EE (%) | DL (%) | ||||
| 0.8 | 20 | 0.5 | 30 | 46.6 | 10.2 | 44.8 | 16.1 |
| 1.3 | 20 | 0.5 | 30 | 66.3 | 23.8 | 55.2 | 20.1 |
| 1.6 | 20 | 0.5 | 30 | 71.6 | 25.5 | 61.6 | 22.1 |
| 1.3 | 10 | 0.5 | 30 | 39.9 | 6.3 | 56.9 | 9.5 |
| 1.3 | 30 | 0.5 | 30 | 83.9 | 51.1 | 64.4 | 39.4 |
| 1.3 | 20 | 0.3 | 30 | - | - | 71.0 | 25.7 |
| 1.3 | 20 | 0.7 | 30 | 56,4 | 20,8 | 46.7 | 16.9 |
| 1.3 | 20 | 0.5 | 50 | 56.4 | 20.8 | 66.6 | 24.1 |
| 1.3 | 20 | 0.5 | 70 | 65.2 | 23.3 | 65.1 | 23.2 |
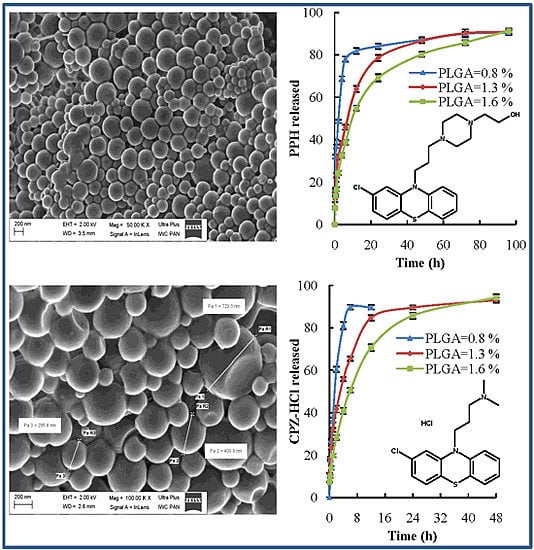
2.4. Morphology of Nanoparticles
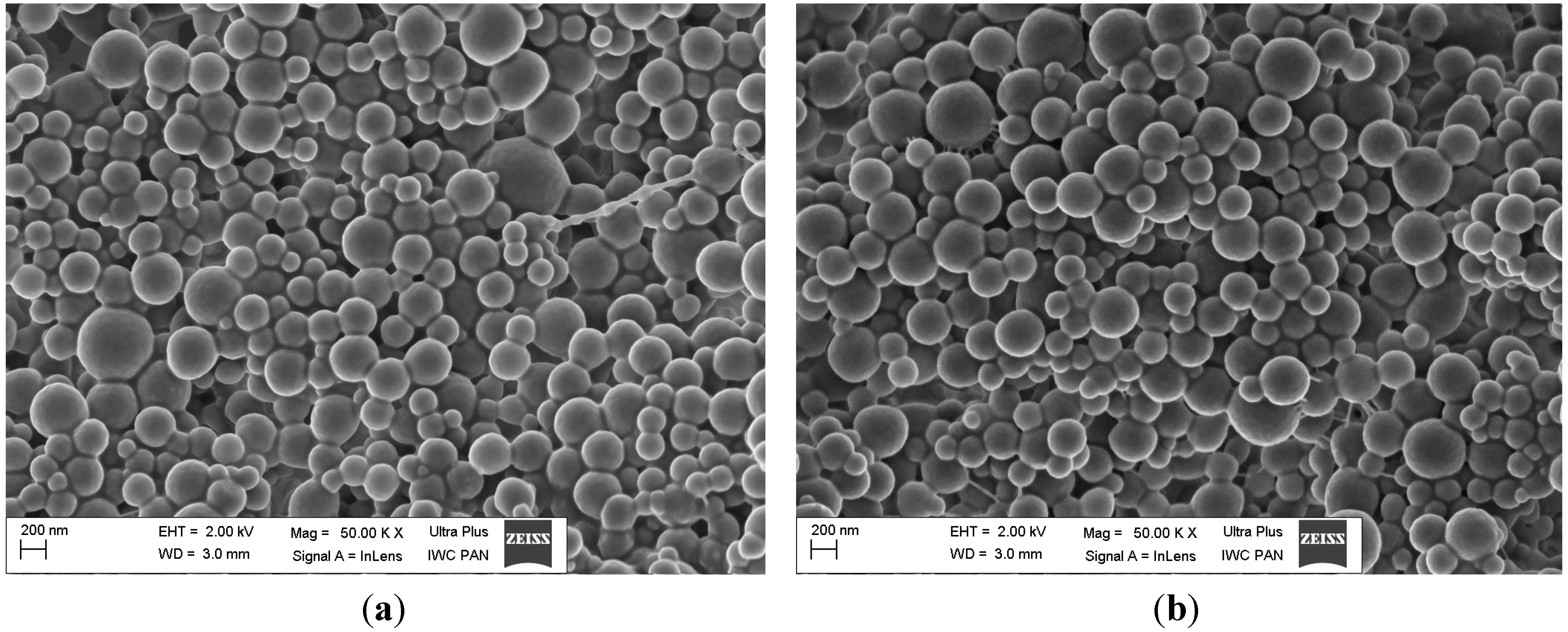


2.5. In Vitro Drug Release


3. Experimental Section
3.1. Chemicals and Reagents
3.2. Preparation of Nanoparticles
3.3. Size Distribution and Polydispersity Index
3.4. Scanning Electron Microscopy
3.5. Assay of Encapsulation
3.6. In Vitro Drug Release
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Cooper, D.L.; Harirforoosh, S. Design and optimization of PLGA-based diclofenac loaded nanoparticles. PLoS One 2014, 9, e87326. [Google Scholar] [CrossRef] [PubMed]
- Zarrabi, A.; Vossoughi, M.; Alemzadeh, I.; Chitsazi, M.R. Monodispersed polymeric nanoparticles fabrication by electrospray atomization. Int. J. Polym. Mater. 2012, 61, 611–626. [Google Scholar] [CrossRef]
- Zhou, Y.Y.; Du, Y.Z.; Wang, L.; Zhou, J.P.; Hu, F.Q. Preparation and pharmacodynamics of stearic acid and poly(lactic-co-glycolic acid) grafted chitosan oligosaccharide micelles for 10-hydroxycamptothecin. Int. J. Pharm. 2010, 393, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Rinaldi, S.; Fortunati, E.; Taddei, M.; Kenny, J.M.; Armentano, I.; Latterini, L. Integrated PLGA-Agnanocomposite systems to control the degradation rate and antibacterial properties. J. Appl. Polym. Sci. 2013, 130, 1185–1193. [Google Scholar] [CrossRef]
- Pascolo, L.; Bortot, B.; Benseny-Cases, N.; Gianoncelli, A.; Tosi, G.; Ruozi, B.; Rizzardi, K.; de Martino, E.; Vandelli, M.; Severini, G.M. Detection of PLG A-based nanoparticles at a single-cell level by synchrotron radiation FTIR spectromicroscopy and correlation with X-ray fluorescence microscopy. Int. J. Nanomed. 2014, 9, 2791–2801. [Google Scholar]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Danhier, F.; Lecouturier, N.; Vromana, B.; Jerome, C.; Marchand-Brynaert, J.; Ferond, O.; Preat, V. Paclitaxelloaded PEGylated PLGA-based nanoparticles: In vitro and in vivo evaluation. J. Control. Release 2009, 133, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Pelczarska, A.; Delie, F.; Domańska, U.; Carrupt, P.; Martel, S. New high throughput screening method for drug release measurements. Eur. J. Pharm. Biopharm. 2013, 85, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Song, C.X.; Labhasetwar, V.; Murphy, H.; Qu, X.; Humphrey, W.R.; Shebuski, R.J.; Levy, R.J. Formulation and characterization of biodegradable nanoparticles for intravascular local drug delivery. J. Control. Release 1997, 43, 197–212. [Google Scholar] [CrossRef]
- Kumar, G.; Sharma, S.; Shafiq, N.; Khuller, G.K.; Malhotra, S. Optimization, in vitro-in vivo evaluation, and short-term torelability of novel levofloxacin-loaded PLGA nanoparticle formulation. J. Pharm. Sci. 2012, 101, 2165–2176. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, P.; Peng, Z.; She, F.H.; Kong, L.X. Microencapsulation of nanoparticles with enhanced drug loading for pH-sensitive oral drug delivery for the treatment of colon cancer. J. Appl. Polym. Sci. 2013, 129, 714–720. [Google Scholar] [CrossRef]
- Mainardes, R.M.; Evangelista, R.C. PLGA nanopartcles containing praziquantel: Effect of formulation variable on size distribution. Int. J. Pharm. 2005, 290, 137–144. [Google Scholar] [CrossRef] [PubMed]
- PIENO. Available online: http://www.parkinsons-information-exchange-network-online.com/drugdb/029.html (accessed on October 2014).
- Samara, M.T.; Cao, H.; Helfer, B.; Davis, J.M.; Leucht, S. Chlorpromazine versus very other antipsychotic for schizophrenia: A systematic review and meta-analysis challenging the dogma of equal efficacy of antipsychotic drugs. Eur. Neuropsychopharmacol. 2014, 24, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Chalikwar, S.S.; Mene, S.B.; Pardeshi, C.V.; Belgamwar, V.S.; Surana, S.J. Self-assembled, chitosan grafted PLGA nanoparticles for intranasal delivery: Design, development and ex vivo characterization. Polym.-Plast. Technol. Eng. 2013, 52, 368–380. [Google Scholar] [CrossRef]
- Meltzer, H.Y.; Massey, B.W. The role of serotonin receptors in the action of atypical antipsychotic drugs. Curr. Opin. Pharmacol. 2011, 11, 59–67. [Google Scholar] [CrossRef] [PubMed]
- Shahnaz, G.; Harlt, M.; Barthelmes, J.; Leithner, K.; Sarti, F.; Hintzen, F.; Rahmat, D.; Salvenmoser, W.; Bernkop-Schnürch, A. Uptake of phenothiazines by the harvested chylomicrons ex vivo model: Influence of sel-nanoemulsifying formulation design. Eur. J. Pharm. Biopharm. 2011, 79, 171–180. [Google Scholar] [CrossRef] [PubMed]
- Aji Alexa, M.R.; Chackoa, A.J.; Josea, S.; Soutob, E.B. Lopinavir loaded solid lipid nanoparticles (SLN) for intestinal lymphatic targeting. Eur. J. Pharm. Sci. 2011, 42, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Domańska, U.; Pelczarska, A.; Pobudkowska, A. Solubility and pKa determination of six structurally related phenothiazines. Int. J. Pharm. 2011, 421, 135–144. [Google Scholar] [CrossRef]
- Quintanar-Guerrero, D.; Fessi, H.; Allémann, E.; Doelker, E. Influence of stabilizing agents and preparatives variables on the formation of poly(dl-lactic acid) nanoparticles by an emulsification-diffusion technique. Int. J. Pharm. 1996, 143, 133–141. [Google Scholar] [CrossRef]
- Murakami, H.; Kobayashi, M.; Takeuchi, H.; Kawashima, Y. Preparation of poly(dl-lactide-co-glycolide) nanoparticles by modified spontaneous emulsification solvent diffusion method. Int. J. Pharm. 1999, 187, 143–152. [Google Scholar] [CrossRef]
- Galindo-Rodriguez, S.; Alleman, E.; Fessi, H.; Doelker, E. Physicochemical parameters associated with nanoparticle formulation in the salting-out emulsification-diffusion and nanoprecipitation methods. Pharm. Res. 2004, 21, 1428–1439. [Google Scholar] [CrossRef]
- Nandi, A.; Khakhar, D.V.; Mehra, A. Coalescence in surfactant-stabilized emulsions subjected to shear flow. Langmuir 2001, 17, 2647–2655. [Google Scholar] [CrossRef]
- Tesch, S.; Schubert, H. Influence of increasing viscosity of the aqueous phase on the short-term stability of protein stabilization emulsion. J. Food Eng. 2002, 52, 305–312. [Google Scholar] [CrossRef]
- Song, X.; Zhao, Y.; Wu, W.; Bi, Y.; Cai, Z.; Chen, Q.; Li, Y.; Hou, S. PLGA nanoparticles simultaneously loaded with vincristine sulfate and verapamil hydrochloride: Systematic study of particle size and drug entrapment efficiency. Int. J. Pharm. 2008, 350, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.-Y.; Lee, J.-Y.; Choi, S.-W.; Jang, Y.; Kim, J.-H. Preparation of PLGA nanoparticles containing estrogen by emulsification-diffusion method. Colloids Surf. A 2001, 182, 123–130. [Google Scholar] [CrossRef]
- Budhian, A.; Siegel, S.J.; Winey, K.I. Haloperidol-loaded PLGA nanoparticles: Systematic study of particle size and drug content. Int. J. Pharm. 2007, 336, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Das, R.K.; Kasoju, N.; Bora, U. Encapsulation of curcumin in alginate-chitosan-pluronic composite nanoparticles for delivery to cancer cells. Nanomedicine 2010, 6, 153–160. [Google Scholar] [CrossRef] [PubMed]
- Fritze, A.; Hens, F.; Kimpfler, A.; Schubert, R.; Peschka-Suss, R. Remote loading of doxorubicin into liposomes driven by a transmembrane phosphate gradient. Biochim. Biophys. Acta 2006, 1758, 1633–1640. [Google Scholar] [CrossRef] [PubMed]
- Dunne, M.M.; Ramtoola, Z.; Corrigan, O.I. Fluphanazine release from biodegradable microparticles: Characterization and modelling of release. J. Microencapsul. 2009, 26, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Domańska, U.; Halayqa, M. Promazine Hydrochloride/PLGA biodegradable nanoparticles formulation and release. J. Phys. Chem. Biophys. 2014, 4:2, 1000143. [Google Scholar]
- Zili, Z.; Sfar, S.; Fessi, H. Preparation and characterization of poly-ε-caprolactone nanoparticles containing griseofulvin. Int. J. Pharm. 2005, 294, 261–267. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chen, C.; Lee, Y. Synthesis of high loading and encapsulation efficient paclitaxel-loaded poly(n-butyl cyanoacrylate) nanoparticles via miniemulsion. Int. J. Pharm. 2007, 338, 267–275. [Google Scholar] [CrossRef] [PubMed]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Halayqa, M.; Domańska, U. PLGA Biodegradable Nanoparticles Containing Perphenazine or Chlorpromazine Hydrochloride: Effect of Formulation and Release. Int. J. Mol. Sci. 2014, 15, 23909-23923. https://doi.org/10.3390/ijms151223909
Halayqa M, Domańska U. PLGA Biodegradable Nanoparticles Containing Perphenazine or Chlorpromazine Hydrochloride: Effect of Formulation and Release. International Journal of Molecular Sciences. 2014; 15(12):23909-23923. https://doi.org/10.3390/ijms151223909
Chicago/Turabian StyleHalayqa, Mohammed, and Urszula Domańska. 2014. "PLGA Biodegradable Nanoparticles Containing Perphenazine or Chlorpromazine Hydrochloride: Effect of Formulation and Release" International Journal of Molecular Sciences 15, no. 12: 23909-23923. https://doi.org/10.3390/ijms151223909
APA StyleHalayqa, M., & Domańska, U. (2014). PLGA Biodegradable Nanoparticles Containing Perphenazine or Chlorpromazine Hydrochloride: Effect of Formulation and Release. International Journal of Molecular Sciences, 15(12), 23909-23923. https://doi.org/10.3390/ijms151223909