Transport of Glial Cell Line-Derived Neurotrophic Factor into Liposomes across the Blood-Brain Barrier: In Vitro and in Vivo Studies
Abstract
:1. Introduction
2. Results
2.1. Characterization of Liposomes
2.2. Evaluation of the BBB Model
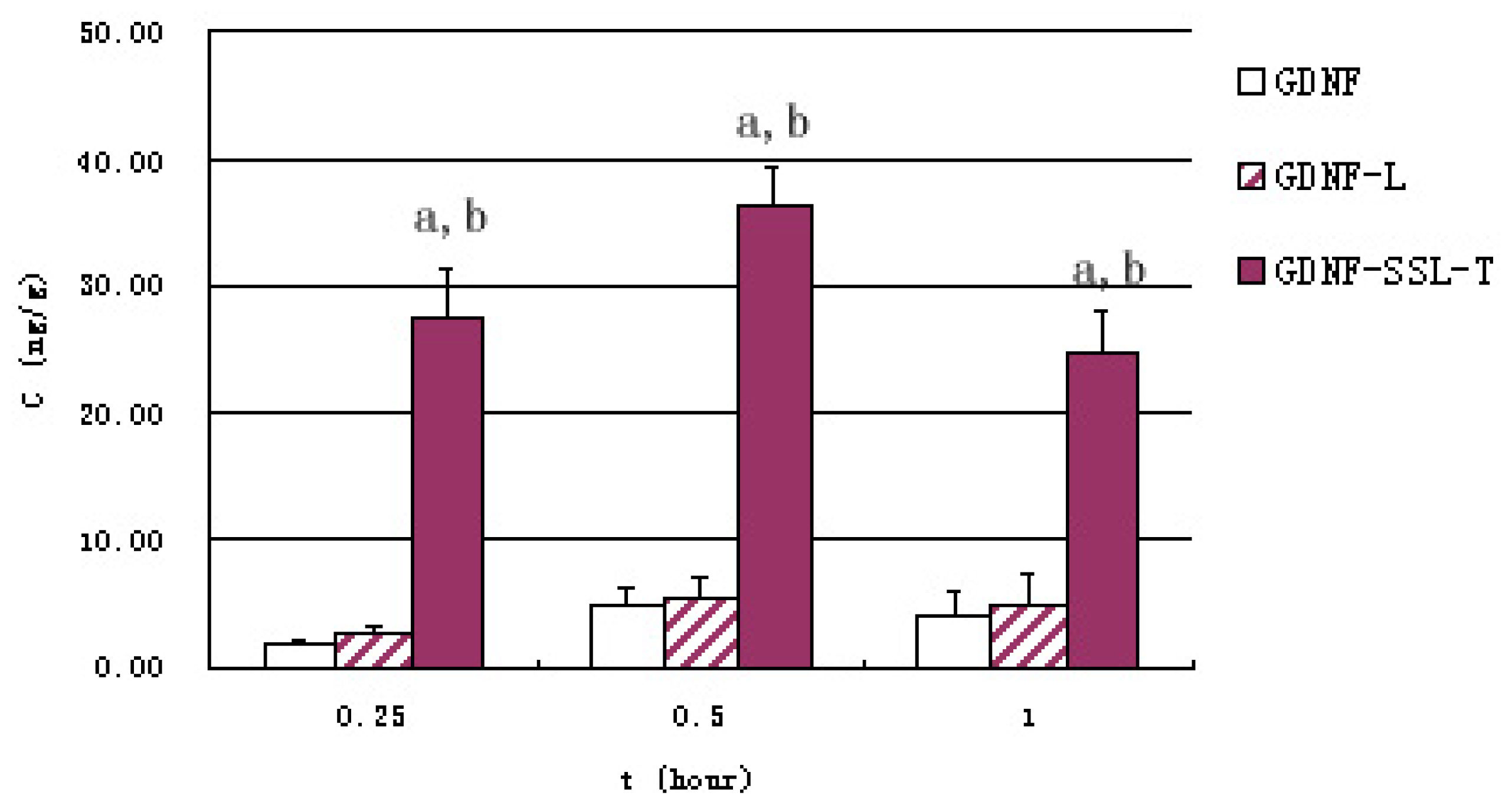
2.3. Transport Measurements of GDNF Liposomes on the in Vitro BBB Model
2.4. Distribution of GDNF Liposomes in Serum and Brain
3. Discussion
4. Materials and Methods
4.1. Materials and Animals
4.2. Preparation and Properties of GDNF Liposomes
4.2.1. FITC-Labeled GDNF (F-GDNF)
4.2.2. Preparation of GDNF Conventional Liposomes (GDNF-L)
4.2.3. Preparation of GDNF Targeted Sterically Stabilized Liposomes (GDNF-SSL-T)
4.2.4. Properties of GDNF Liposomes
4.3. Establishment and Evaluation of the in Vitro BBB Model
4.3.1. Primary Culture of Rat Brain Capillary Endothelial Cells (BCECs) and Astrocytes (ACs)
4.3.2. The Development of an in Vitro Model of the BBB
4.4. The Permeability of GDNF Liposomes on the BBB Model
4.5. The Transporting Ability of GDNF Liposomes across the BBB in Vivo
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF, and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar]
- Lin, L.F.; Doherty, D.H.; Lile, J.D.; Bektesh, S.; Collins, F. GDNF: A glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science 1993, 260, 1130–1132. [Google Scholar]
- Nosrat, C.A.; Tomac, A.; Lindqvist, E.; Lindskog, S.; Humpel, C.; Stromberg, I.; Ebendal, T.; Hoffer, B.J.; Olson, L. Cellular expression of GDNF mRNA suggests multiple functions inside and outside the nervous system. Cell Tissue Res. 1996, 286, 191–207. [Google Scholar]
- Barroso-Chinea, P.; Cruz-Muros, I.; Aymerich, M.S.; Rodriguez-Diaz, M.; Afonso-Oramas, D.; Lanciego, J.L.; González-Hernández, T. Striatal expression of GDNF and differential vulnerability of midbrain dopaminergic cells. Eur. J. Neurosci. 2005, 21, 1815–1827. [Google Scholar]
- Pascual, A.; Hidalgo-Figueroa, M.; Piruat, J.I.; Pintado, C.O.; Gomez-Diaz, R.; Lopez-Barneo, J. Absolute requirement of GDNF for adult catecholaminergic neuron survival. Nat. Neurosci. 2008, 11, 755–761. [Google Scholar] [Green Version]
- Beck, K.D.; Valverde, J.; Alexi, T.; Poulsen, K.; Moffat, B.; Vandlen, R.A.; Rosenthal, A.; Hefti, F. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature 1995, 373, 339–341. [Google Scholar]
- Tomac, A.; Lindqvist, E.; Lin, L.F.; Ogren, S.O.; Young, D.; Hoffer, B.J.; Olson, L. Protection and repair of the nigrostriatal dopaminergic system by GDNF in vivo. Nature 1995, 373, 335–339. [Google Scholar]
- Moore, M.W.; Klein, R.D.; Farinas, I.; Sauer, H.; Armanini, M.; Phillips, H.; Reichardt, L.F.; Ryan, A.M.; Carver-Moore, K.; Rosenthal, A. Renal and neuronal abnormalities in mice lacking GDNF. Nature 1996, 382, 76–79. [Google Scholar]
- Ledda, F.; Paratcha, G.; Sandoval-Guzman, T.; Ibanez, C.F. GDNF and GFRalpha1 promote formation of neuronal synapses by ligand-induced cell adhesion. Nat. Neurosci. 2007, 10, 293–300. [Google Scholar]
- Hirsch, E.C.; Hunot, S.; Faucheux, B. Dopaminergic neurons degenerate by apoptosis in Parkinson’s disease. Mov. Disord. 1999, 14, 383–385. [Google Scholar]
- Sinclair, S.R.; Svendsen, C.N.; Torres, E.M.; Martin, D.; Fawcett, J.W.; Dunnett, S.B. GDNF enhances dopaminergic cell survival and fibre outgrowth in embryonic nigral grafts. Neuroreport 1996, 7, 2547–2552. [Google Scholar]
- Björklund, A.; Kirik, D.; Rosenblad, C.; Georgievska, B.; Lundberg, C.; Mandel, R.J. Towards a neuroprotective gene therapy for Parkinson disease: Use of adenovirus, AAV and lentivirus vectors for gene transfer of GDNF to the nigrostriatal system in the rat Parkinson model. Brain Res. 2000, 886, 82–98. [Google Scholar]
- Gash, D.M.; Zhang, Z.; Ovadia, A.; Cass, W.A.; Yi, A.; Simmerman, L.; Russell, D.; Martin, D.; Lapchak, P.A.; Collins, F.; et al. Functional recovery in parkinsonian monkeys treated with, GDNF. Nature 1996, 380, 252–255. [Google Scholar]
- Yang, W.H.; Yang, C.; Xue, Y.Q.; Lu, T.; Reiser, J.; Zhao, L.R.; Duan, W.M. Regulated expression of lentivirus-mediated GDNF in human bone marrow-derived mesenchymal stem cells and its neuroprotection on dopaminergic cells in vitro. PLoS One 2013, 22, e64389. [Google Scholar]
- Garbayo, E.; Montero-Menei, C.N.; Ansorena, E.; Lanciego, J.L.; Aymerich, M.S.; Blanco-Prieto, M.J. Effective GDNF brain delivery using microspheres—A promising strategy for Parkinson’s Disease. J. Control. Release 2009, 135, 119–126. [Google Scholar]
- Fletcher, A.M.; Kowalczyk, T.H.; Padegimas, L.; Cooper, M.J.; Yurek, D.M. Transgene expression in the striatum following intracerebral injections of DNA nanoparticles encoding for human glial cell line-derived neurotrophic factor. Neu-roscience 2011, 194, 220–226. [Google Scholar]
- Torchilin, V.P. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005, 4, 145–160. [Google Scholar]
- Ramos-Cabrer, P.; Campos, F. Liposomes and nanotechnology in drug development: Focus on neurological targets. Int. J. Nanomed. 2013, 8, 951–960. [Google Scholar]
- Cecchelli, R.; Berezowski, V.; Lundquist, S.; Culot, M.; Renftel, M.; Dehouck, M.P.; Fenart, L. Modelling of the blood-brain barrier indrug discovery and development. Nat. Rev. Drug Discov. 2007, 6, 650–661. [Google Scholar]
- Liu, W.Y.; Wang, Z.B.; Zhang, L.C.; Wei, X.; Li, L. Tight junction in blood-brain barrier: An overview of structure regulation and regulator substances. CNS Neurosci. Ther. 2012, 18, 609–615. [Google Scholar]
- Pardridge, W.M. BBB-Genomics: Creating new openings for brain-drug targeting. Drug Discov. Today 2001, 6, 381–383. [Google Scholar]
- Markoutsa, E.; Pampalakis, G.; Niarakis, A.; Romero, I.A.; Weksler, B.; Couraud, P.O.; Antimisiaris, S.G. Uptake and permeability studies of BBB-targeting immunoliposomes using the hCMEC/D3 cell line. Eur. J. Pharm. Biopharm. 2011, 77, 265–274. [Google Scholar]
- Zhang, Y.; Schlachetzki, F.; Zhang, Y.F.; Boado, R.J.; Pardridge, W.M. Normalization of striatal tyrosine hydroxylase and reversal of motor impairment in experimental parkinsonism with intravenous nonviral gene therapy and a brain-specific promoter. Hum. Gene Ther. 2004, 15, 339–350. [Google Scholar]
- Chen, H.; Tang, L.; Qin, Y.; Yin, Y.; Tang, J.; Tang, W.; Sun, X.; Zhang, Z.; Liu, J.; He, Q. Lactoferrin-modified procationic liposomes as a novel drug carrier for brain delivery. Eur. J. Pharm. Sci. 2010, 40, 94–102. [Google Scholar]
- Qin, Y.; Fan, W.; Chen, H.; Yao, N.; Tang, W.; Tang, J.; Yuan, W.; Kuai, R.; Zhang, Z.; Wu, Y.; et al. In vitro and in vivo investigation of glucose-mediated brain-targeting liposomes. J. Drug Target. 2010, 18, 536–549. [Google Scholar]
- Demeuse, P.; Kerkhofs, A.; Struys-Ponsar, C.; Knoops, B.; Remacle, C.; van den Bosch de Aguilar, P. Compartmentalized coculture of rat brain endothelial cells and astrocytes: A syngenic model to study the blood-brain barrier. J. Neurosci. Methods 2002, 121, 21–31. [Google Scholar]
- Xie, Y.; Ye, L.; Zhang, X.; Cui, W.; Lou, J.; Nagai, T.; Hou, X. Transport of nerve growth factor encapsulated into liposomes across the blood-brain barrier: in vitro and in vivo studies. J. Control. Release 2005, 105, 106–119. [Google Scholar]
- Senior, J.; Delgado, C.; Fisher, D.; Tilcock, C.; Gregoriadis, G. Influence of surface hydrophilicity of liposomes on their interaction with plasma protein and clearance from the circulation: Studies with PEGcoated vesicles. Biochim. Biophys. Acta 1991, 1062, 77–82. [Google Scholar]
- Klibanov, A.L.; Maruyama, K.; Torchilin, V.P.; Huang, L. Amphipatic PEG effectively prolong the circulation time of liposomes. FEBS Lett. 1990, 268, 235–237. [Google Scholar]
- Ying, X.; Wen, H.; Lu, W.L.; Du, J.; Guo, J.; Tian, W.; Men, Y.; Zhang, Y.; Li, R.J.; Yang, T.Y.; et al. Dual-targeting daunorubicin liposomes improve the therapeutic efficacy of brain glioma in animals. J. Control. Release 2010, 141, 183–192. [Google Scholar]
- Doctrow, S.R.; Abelleira, S.M.; Curry, L.A.; Heller-Harrison, R.; Kozarich, J.W.; Malfroy, B.; McCarroll, L.A.; Morgan, K.G.; Morrow, A.R.; Musso, G.F.; et al. The bradykinin analog RMP-7 increases intracellular free calcium levels in rat brain microvascullar endothelial cell. J. Pharmacol. Exp. Ther. 1994, 271, 229– 237. [Google Scholar]
- Bartus, R.T.; Elliott, P.J.; Dean, R.L.; Hayward, N.J.; Nagle, T.L.; Huff, M.R.; Snodgrass, P.A.; Blunt, D.G. Controlled modulation of BBB permeability using the bradykinin agonist RMP-7. Exp. Neurol. 1996, 142, 14–28. [Google Scholar]
- Szoka, F., Jr; Papahadjopoulos, D. Procedure for preparation of liposomes with large internal aqueous space and high capture by reverse-phase evaporation. Proc. Natl. Acad. Sci. USA 1978, 75, 4194–4198. [Google Scholar]
- Zhang, X.; Xie, J.; Li, S.; Wang, X.; Hou, X. The study on brain targeting of the amphotericin B liposomes. J. Drug Target. 2003, 11, 117–122. [Google Scholar]
- Lu, W.; Zhang, Y.; Tan, Y.Z.; Hu, K.L.; Jiang, X.G.; Fu, S.K. Cationic albumin-conjugated pegylated nanoparticles as novel drug carrier for brain delivery. J. Control. Release 2005b, 107, 428–448. [Google Scholar]
- Lu, W.; Tan, Y.Z.; Hu, K.L.; Jiang, X.G. Cationic albumin conjugated pegylated nanoparticle with its transcytosis ability and little toxicity against blood-brain barrier. Int. J. Pharm. 2005a, 295, 247–260. [Google Scholar]
- Kurakhmaeva, K.B.; Djindjikhashvili, I.A.; Petrov, V.E.; Balabanyan, V.U.; Voronina, T.A.; Trofimov, S.S.; Kreuter, J.; Gelperina, S.; Begley, D.; Alyautdin, R.N. Brain targeting of nerve growth factor using poly (butyl cyanoacrylate) nanoparticles. J. Drug Target. 2009, 17, 564–574. [Google Scholar]


| Mean size (nm) | Encapsulation efficiency (%) | Recovery efficiency (%) | |
|---|---|---|---|
| GDNF-L | 85.65 ± 0.75 | 33.25 ± 0.92 | 99.15 ± 5.81 |
| GDNF-SSL-T | 81.50 ± 0.66 | 37.46 ± 1.75 | 97.38 ± 4.09 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wu, S.; Li, G.; Li, X.; Lin, C.; Yu, D.; Luan, S.; Ma, C. Transport of Glial Cell Line-Derived Neurotrophic Factor into Liposomes across the Blood-Brain Barrier: In Vitro and in Vivo Studies. Int. J. Mol. Sci. 2014, 15, 3612-3623. https://doi.org/10.3390/ijms15033612
Wu S, Li G, Li X, Lin C, Yu D, Luan S, Ma C. Transport of Glial Cell Line-Derived Neurotrophic Factor into Liposomes across the Blood-Brain Barrier: In Vitro and in Vivo Studies. International Journal of Molecular Sciences. 2014; 15(3):3612-3623. https://doi.org/10.3390/ijms15033612
Chicago/Turabian StyleWu, Shaoling, Guoqi Li, Xiao Li, Caina Lin, Ding Yu, Shuo Luan, and Chao Ma. 2014. "Transport of Glial Cell Line-Derived Neurotrophic Factor into Liposomes across the Blood-Brain Barrier: In Vitro and in Vivo Studies" International Journal of Molecular Sciences 15, no. 3: 3612-3623. https://doi.org/10.3390/ijms15033612




