Effects of Estradiol and Methoxychlor on Leydig Cell Regeneration in the Adult Rat Testis
Abstract
:1. Introduction
2. Results
2.1. General Reproductive Toxicology
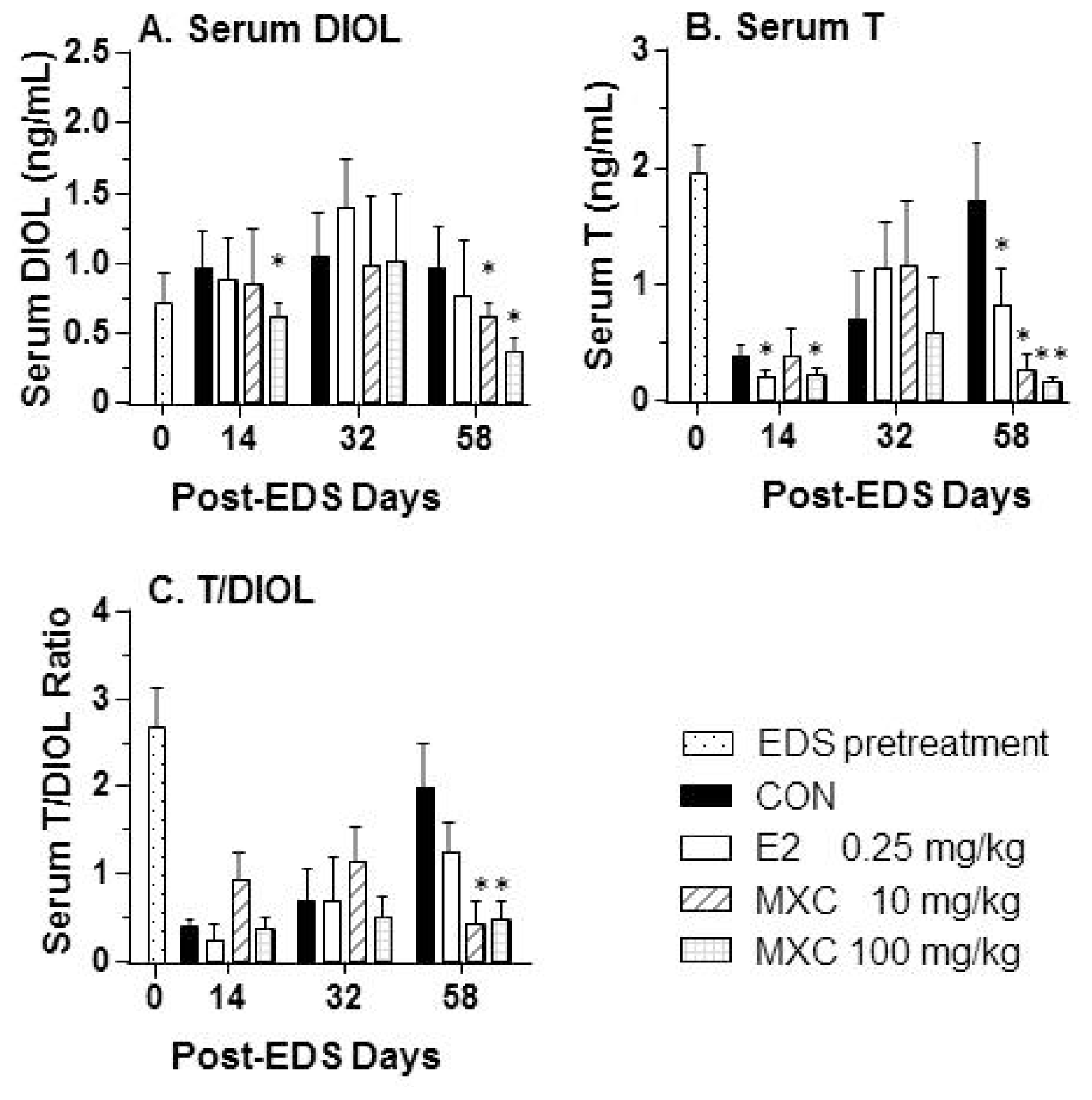
2.2. Serum Testosterone and DIOL Levels
2.3. Effects of E2 and MXC on Leydig Cell Number and Maturity, as well as Spermatogenesis
2.4. Effects of E2 and MXC on Genes Related to Leydig Cell Regeneration
2.5. Androgen Biosynthetic Enzyme Activities
2.6. Effects of E2 and MXC on Genes in the Pituitary
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Animal Treatment
4.3. Determination of Leydig Cell Number
4.4. Quantitative PCR
4.5. Homogenization and Protein Content Assay
4.6. Enzyme Assay
4.7. Statistical Analysis
5. Conclusions
Acknowledgments
Conflicts of Interest
- Author ContributionsXiaokun Li and Ren-Shan Ge conceived and designed the study. Bingbing Chen, Dongxin Chen, Zheli Jiang, Jingyang Li, Siwen Liu, Yaoyao Dong, and Wenwen Yao performed the experiments. Bingbing Chen wrote the paper. Bingbing Chen, Renshan Ge and Benson Akingbemi reviewed and edited the manuscript. All authors read and approved the manuscript.
References
- Akingbemi, B.T.; Ge, R.S.; Klinefelter, G.R.; Gunsalus, G.L.; Hardy, M.P. A metabolite of methoxychlor, 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane, reduces testosterone biosynthesis in rat leydig cells through suppression of steady-state messenger ribonucleic acid levels of the cholesterol side-chain cleavage enzyme. Biol. Reprod 2000, 62, 571–578. [Google Scholar]
- Al-Jamal, J.H.; Dubin, N.H. The effect of raloxifene on the uterine weight response in immature mice exposed to 17β-estradiol, 1,1,1-trichloro-2,2-bis(p-chlorophenyl)ethane, and methoxychlor. Am. J. Obstet. Gynecol 2000, 182, 1099–1102. [Google Scholar]
- Lafuente, A.; Marquez, N.; Pousada, Y.; Pazo, D.; Esquifino, A.I. Possible estrogenic and/or antiandrogenic effects of methoxychlor on prolactin release in male rats. Arch. Toxicol 2000, 74, 270–275. [Google Scholar]
- Kapoor, I.P.; Metcalf, R.L.; Nystrom, R.F.; Sangha, G.K. Comparative metabolism of methoxychlor, methiochlor, and ddt in mouse, insects, and in a model ecosystem. J. Agric. Food Chem 1970, 18, 1145–1152. [Google Scholar]
- Hu, G.X.; Zhao, B.; Chu, Y.; Li, X.H.; Akingbemi, B.T.; Zheng, Z.Q.; Ge, R.S. Effects of methoxychlor and 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane on 3β-hydroxysteroid dehydrogenase and 17β-hydroxysteroid dehydrogenase-3 activities in human and rat testes. Int. J. Androl 2011, 34, 138–144. [Google Scholar]
- Guo, J.; Deng, H.; Li, H.; Zhu, Q.; Zhao, B.; Chen, B.; Chu, Y.; Ge, R.S. Effects of methoxychlor and its metabolite 2,2-bis(p-hydroxyphenyl)-1,1,1-trichloroethane on 11β-hydroxysteroid dehydrogenase activities in vitro. Toxicol. Lett. 2013, 218, 18–23. [Google Scholar]
- Abney, T.O. The potential roles of estrogens in regulating leydig cell development and function: A review. Steroids 1999, 64, 610–617. [Google Scholar]
- Teerds, K.J. Regeneration of leydig cells after depletion by eds: A model for postnatal leydig cell renewal. In The Leydig Cell; Payne, A.H., Hardy, M.P., Russell, L.D., Eds.; Cache River Press: Vienna, IL, USA, 1996; pp. 203–220. [Google Scholar]
- Kerr, J.B.; Sharpe, R.M. Stimulatory effect of follicle-stimulating hormone on rat leydig cells. A morphometric and ultrastructural study. Cell Tissue Res 1985, 239, 405–415. [Google Scholar]
- Guo, J.; Zhou, H.; Su, Z.; Chen, B.; Wang, G.; Wang, C.Q.; Xu, Y.; Ge, R.S. Comparison of cell types in the rat leydig cell lineage after ethane dimethanesulfonate treatment. Reproduction 2013, 145, 371–380. [Google Scholar]
- Teerds, K.J.; de Rooij, D.G.; Rommerts, F.F.; van den Hurk, R.; Wensing, C.J. Stimulation of the proliferation and differentiation of leydig cell precursors after the destruction of existing leydig cells with ethane dimethyl sulphonate (EDS) can take place in the absence of l h. J. Androl 1989, 10, 472–477. [Google Scholar]
- Teerds, K.J.; de Rooij, D.G.; Rommerts, F.F.; Wensing, C.J. The regulation of the proliferation and differentiation of rat leydig cell precursor cells after eds administration or daily hcg treatment. J. Androl 1988, 9, 343–351. [Google Scholar]
- Lin, H.; Lian, Q.Q.; Hu, G.X.; Jin, Y.; Zhang, Y.; Hardy, D.O.; Chen, G.R.; Lu, Z.Q.; Sottas, C.M.; Hardy, M.P.; et al. In utero and lactational exposures to diethylhexyl-phthalate affect two populations of leydig cells in male long-evans rats. Biol. Reprod 2009, 80, 882–888. [Google Scholar]
- Abney, T.O.; Myers, R.B. 17β-estradiol inhibition of leydig cell regeneration in the ethane dimethylsulfonate-treated mature rat. J. Androl 1991, 12, 295–304. [Google Scholar]
- Kan, X.; Zhang, X.Y.; Wang, Z.; Dong, J.; Wang, M.; Hu, G.X. Toxicokinetics of methoxychlor and its metabolite 2′2-bis-(p-hydroxyphenyl)-1′1′1-trichloroethane in rats. Chin. J. Pharmacol. Toxicol 2011, 25, 474–478. [Google Scholar]
- Petroff, B.K.; Mizinga, K.M. Pharmacokinetics of ovarian steroids in sprague-dawley rats after acute exposure to 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD). Reprod. Biol 2003, 3, 131–141. [Google Scholar]
- Ge, R.S.; Dong, Q.; Sottas, C.M.; Chen, H.; Zirkin, B.R.; Hardy, M.P. Gene expression in rat leydig cells during development from the progenitor to adult stage: A cluster analysis. Biol. Reprod 2005, 72, 1405–1415. [Google Scholar]
- Gnessi, L.; Basciani, S.; Mariani, S.; Arizzi, M.; Spera, G.; Wang, C.; Bondjers, C.; Karlsson, L.; Betsholtz, C. Leydig cell loss and spermatogenic arrest in platelet-derived growth factor (pdgf)-a-deficient mice. J. Cell Biol 2000, 149, 1019–1026. [Google Scholar]
- Mauduit, C.; Goddard, I.; Besset, V.; Tabone, E.; Rey, C.; Gasnier, F.; Dacheux, F.; Benahmed, M. Leukemia inhibitory factor antagonizes gonadotropin induced-testosterone synthesis in cultured porcine leydig cells: Sites of action. Endocrinology 2001, 142, 2509–2520. [Google Scholar]
- Zhang, F.P.; Poutanen, M.; Wilbertz, J.; Huhtaniemi, I. Normal prenatal but arrested postnatal sexual development of luteinizing hormone receptor knockout (lurko)mice. Mol. Endocrinol 2001, 15, 172–183. [Google Scholar]
- Nakamura, D.; Yanagiba, Y.; Duan, Z.; Ito, Y.; Okamura, A.; Asaeda, N.; Tagawa, Y.; Li, C.; Taya, K.; Zhang, S.Y.; et al. Bisphenol a may cause testosterone reduction by adversely affecting both testis and pituitary systems similar to estradiol. Toxicol. Lett 2010, 194, 16–25. [Google Scholar]
- Gray, L.E., Jr.; Ostby, J.; Ferrell, J.; Rehnberg, G.; Linder, R.; Cooper, R.; Goldman, J.; Slott, V.; Laskey, J. A dose-response analysis of methoxychlor-induced alterations of reproductive development and function in the rat. Fundam. Appl. Toxicol 1989, 12, 92–108. [Google Scholar]
- Gaido, K.W.; Maness, S.C.; McDonnell, D.P.; Dehal, S.S.; Kupfer, D.; Safe, S. Interaction of methoxychlor and related compounds with estrogen receptor α and β, and androgen receptor: Structure-activity studies. Mol. Pharmacol 2000, 58, 852–858. [Google Scholar]
- Ge, R.-S.; Dong, Q.; Niu, E.-M.; Sottas, C.M.; Hardy, D.O.; Catterall, J.F.; Latif, S.A.; Morris, D.J.; Hardy, M.P. 11β-hydroxysteroid dehydrogenase 2 in rat leydig cells: Its role in blunting glucocorticoid action at physiological levels of substrate. Endocrinology 2005, 146, 2657–2664. [Google Scholar]
- Lin, H.; Ge, R.S.; Chen, G.R.; Hu, G.X.; Dong, L.; Lian, Q.Q.; Hardy, D.O.; Sottas, C.M.; Li, X.K.; Hardy, M.P. Involvement of testicular growth factors in fetal leydig cell aggregation after exposure to phthalate in utero. Proc. Natl. Acad. Sci. USA 2008, 105, 7218–7222. [Google Scholar]
- Ye, L.; Zhao, B.; Hu, G.; Chu, Y.; Ge, R.S. Inhibition of human and rat testicular steroidogenic enzyme activities by bisphenol a. Toxicol. Lett 2011, 207, 137–142. [Google Scholar]
- Rommerts, F.F.; King, S.R.; Span, P.N. Implications of progesterone metabolism in ma-10 cells for accurate measurement of the rate of steroidogenesis. Endocrinology 2001, 142, 5236–5242. [Google Scholar]
- Ge, R.S.; Hardy, M.P. Variation in the end products of androgen biosynthesis and metabolism during postnatal differentiation of rat leydig cells. Endocrinology 1998, 139, 3787–3795. [Google Scholar]






| Treatment | Control | Estradiol | Methoxychlor | |
|---|---|---|---|---|
| Dosage (mg/kg) | 0 | 0.25 | 10 | 100 |
| Body weight (g) | ||||
| 14 days post-EDS | 309.2 ± 4. 46 | 322.5 ± 4.88 | 360.2 ± 9.05 ** | 327.8 ± 2.24 |
| 32 days post-EDS | 375.7 ± 5.75 | 354.3 ± 11.49 | 389.0 ± 9.09 | 380.7 ± 5.10 |
| 58 days post-EDS | 424.3 ± 10.79 | 416.8 ± 19.01 | 413.5 ± 9.38 | 408.5 ± 6.97 |
| Testis weight (g) | ||||
| 14 days post-EDS | 0.751 ± 0.008 | 0.779 ± 0.065 | 0.919 ± 0.132 | 0.875 ± 0.067 |
| 32 days post-EDS | 0.896 ± 0.107 | 1.185 ± 0.133 | 1.186 ± 0.167 | 0.914 ± 0.149 |
| 58 days post-EDS | 1.124 ± 0.099 | 1.448 ± 0.153 | 1.228 ± 0.119 | 1.136 ± 0.108 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chen, B.; Chen, D.; Jiang, Z.; Li, J.; Liu, S.; Dong, Y.; Yao, W.; Akingbemi, B.; Ge, R.; Li, X. Effects of Estradiol and Methoxychlor on Leydig Cell Regeneration in the Adult Rat Testis. Int. J. Mol. Sci. 2014, 15, 7812-7826. https://doi.org/10.3390/ijms15057812
Chen B, Chen D, Jiang Z, Li J, Liu S, Dong Y, Yao W, Akingbemi B, Ge R, Li X. Effects of Estradiol and Methoxychlor on Leydig Cell Regeneration in the Adult Rat Testis. International Journal of Molecular Sciences. 2014; 15(5):7812-7826. https://doi.org/10.3390/ijms15057812
Chicago/Turabian StyleChen, Bingbing, Dongxin Chen, Zheli Jiang, Jingyang Li, Shiwen Liu, Yaoyao Dong, Wenwen Yao, Benson Akingbemi, Renshan Ge, and Xiaokun Li. 2014. "Effects of Estradiol and Methoxychlor on Leydig Cell Regeneration in the Adult Rat Testis" International Journal of Molecular Sciences 15, no. 5: 7812-7826. https://doi.org/10.3390/ijms15057812




