Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells
Abstract
:1. Introduction
2. Results and Discussion
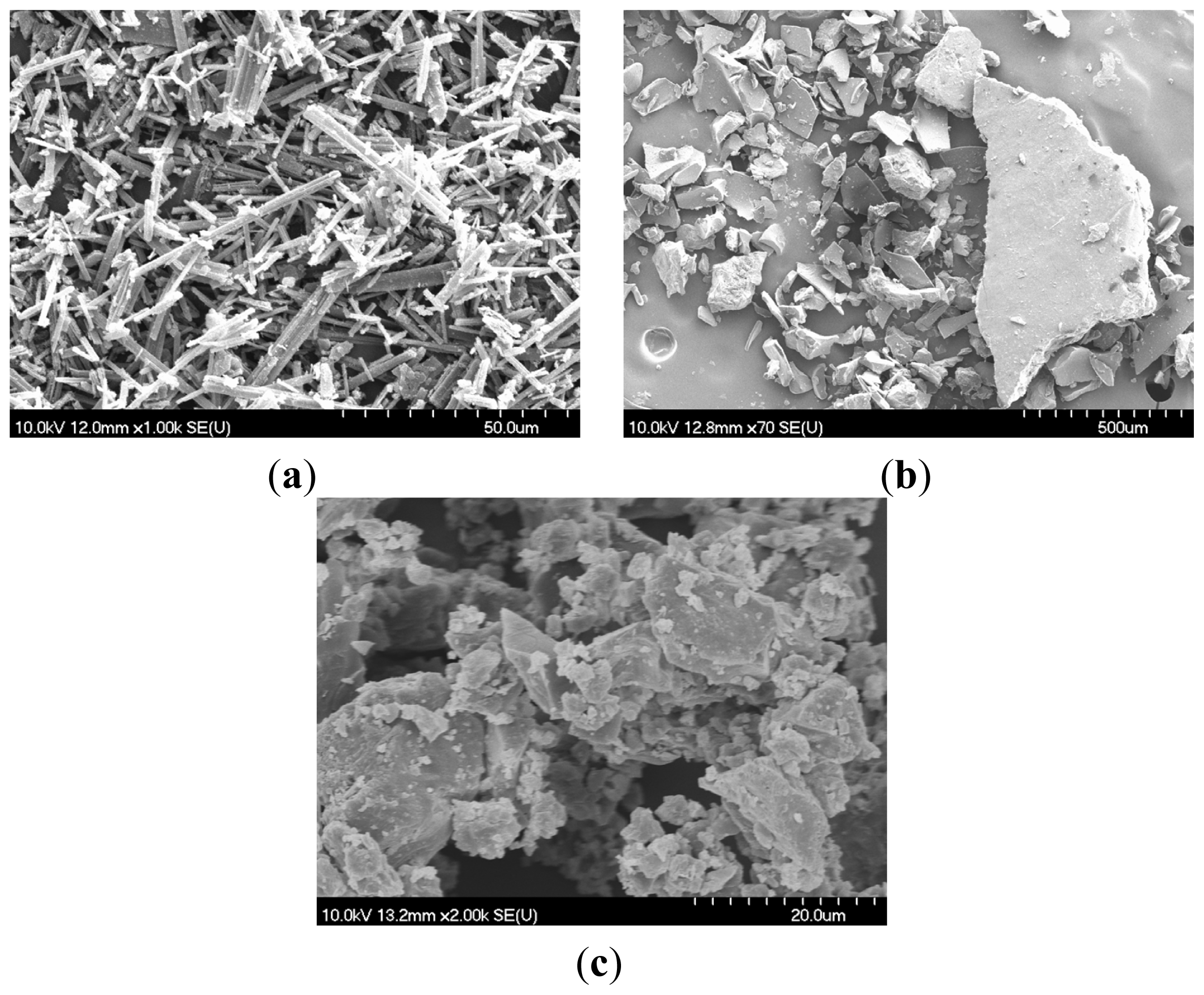
2.1. Scanning Electron Microscopy (SEM)
2.2. Differential Scanning Calorimetry (DSC)
2.3. X-ray Analysis
2.4. MTT Assay
2.5. Cell Cycle
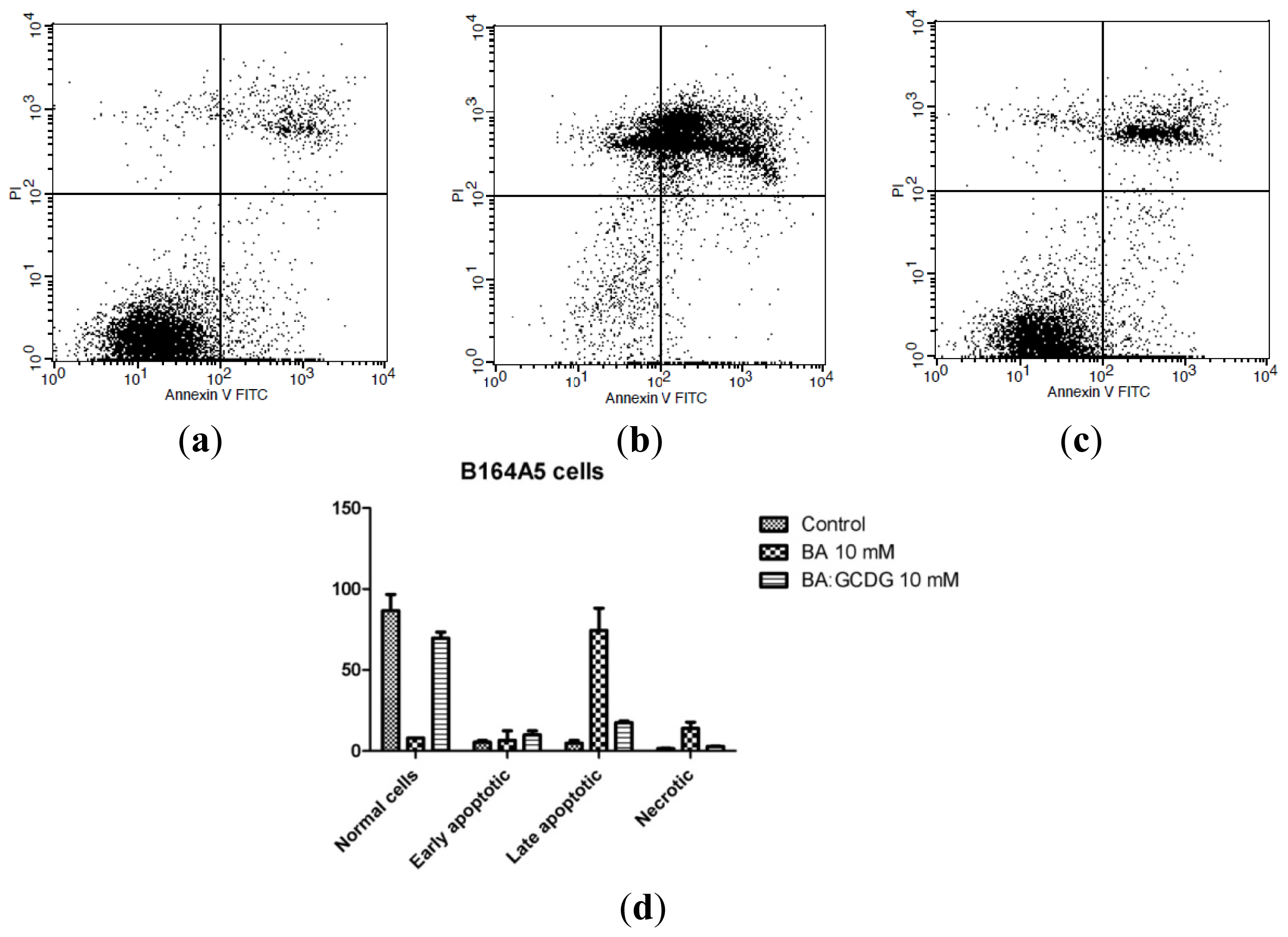
2.6. Annexin V-FITC-PI Double Staining Assay
2.7. In Vivo Experiments
3. Experimental Section
3.1. Preparation of Complexes
3.2. Scanning Electron Microscopy
3.3. Differential Scanning Calorimetry
3.4. X-ray Diffraction
3.5. Isolation of Metastatic Cells
3.6. MTT Assay
3.7. Cell Cycle
3.8. Annexin V/PI Assay
3.9. Statistical Analysis
3.10. Ethics Statement
3.11. Animal Studies
3.12. Non-Invasive Skin Measurements
3.13. Histology
4. Conclusions
Supplementary Information
ijms-15-08235-s001.pdfAcknowledgments
Conflicts of Interest
- Author ContributionsCS designed the entire study, participated in the physico-chemical tests and conceived the draft; CD, IZ, FB4 contributed to the in vitro study and interpreted the results; GS-B, DC, SC and CAD participated to the animal study and writing the article; FB1 and RA participated in the physico-chemical tests; SA performed histological analysis; TO and PM participated to the animal study and contributed to the final form of the manuscript.
References
- Pisha, E.; Chai, H.; Lee, I.-S.; Chagwedera, T.E.; Farnsworth, N.R.; Cordell, G.A.; Beecher, C.W.W.; Fong, H.H.S.; Douglas Kinghorn, A.; Brown, D.M.; et al. Discovery of betulinic acid as a selective inhibitor of human melanoma that functions by induction of apoptosis. Nat. Med 1995, 1, 1046–1051. [Google Scholar]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic acid, a natural compound with potent anticancer effects. Anticancer Drugs 2010, 21, 215–227. [Google Scholar]
- Şoica, C.; Peev, C.; Dehelean, C.; Aluas, M.; Zupko, I.; Kasa, P.; Alexa, E. Physico-chemical comparison study of betulinic acid, betulin and birch bark extract and in vitro investigation of their cytotoxic effects towards skin epidermoid carcinoma (A431), breast carcinoma (MCF7) and cervix adenocarcinoma (HeLa) cell-lines. Nat. Prod. Res 2012, 26, 968–974. [Google Scholar]
- Zuco, V.; Supino, R.; Righetti, S.C.; Cleris, L.; Marchesi, E.; Gambacorti-Passerini, C.; Formelli, F. Selective cytotoxicity of betulinic acid on tumor cell lines, but not on normal cells. Cancer Lett 2002, 175, 17–25. [Google Scholar]
- Drag, M.; Surowiak, P.; Drag-Zalesinska, M.; Dietel, M.; Lage, H.; Oleksyszyn, J. Comparision of the cytotoxic effects of birch bark extract, betulin and betulinic acid towards human gastric carcinoma and pancreatic carcinoma drug-sensitive and drug-resistant cell lines. Molecules 2009, 14, 1639–1651. [Google Scholar]
- Rieber, M.; Strasberg Rieber, M. Induction of p53 without increase in p21WAF1 in betulinic acid-mediated cell death is preferential for human metastatic melanoma. DNA Cell Biol 1998, 17, 399–406. [Google Scholar]
- Sawada, N.; Kataoka, K.; Kondo, K.; Arimochi, H.; Fujino, H.; Takahashi, Y.; Miyoshi, T.; Kuwahara, T.; Monden, Y.; Ohnishi, Y. Betulinic acid augments the inhibitory effects of vincristine on growth and lung metastasis of B16F10 melanoma cells in mice. Br. J. Cancer 2004, 90, 1672–1678. [Google Scholar]
- Cichewicz, R.H.; Kouzi, S.A. Chemistry, biological activity, and chemotherapeutic potential of betulinic acid for the prevention and treatment of cancer and HIV infection. Med. Res. Rev 2004, 24, 90–114. [Google Scholar]
- Şoica, C.M.; Peev, C.I.; Ciurlea, S.; Ambrus, R.; Dehelean,, C. Physico-chemical and toxicological evaluations of betulin and betulinic acid interactions with hydrophilic cyclodextrins. Farmacia 2010, 58, 611–619. [Google Scholar]
- Dehelean, C.A.; Soica, C.; Peev, C.; Ciurlea, S.; Feflea, S.; Kasa, P., Jr. A pharmaco-toxicological evaluation for betulinic acid mixed with hydroxipropilgamma cyclodextrin on in vitro and in vivo models. Farmacia 2011, 59, 51–59. [Google Scholar]
- Dehelean, C.; Şoica, C.; Peev, C.; Gruia, A.T.; Şeclaman, E. Physico-chemical and molecular analysis of antitumoral pentacyclic triterpenes in complexation with gamma-cyclodextrin. Rev. Chim 2008, 59, 887–890. [Google Scholar]
- Şoica, C.; Dehelean, C.; Peev, C.; Coneac, G.; Gruia, A.T. Complexation with hydroxipropil γ cyclodextrin of some pentacyclic triterpenes. Characterisation of their binary products. Farmacia 2008, 56, 182–190. [Google Scholar]
- Iyer, A.K.; He, J.; Amiji, M.M. Image-guided nanosystems for targeted delivery in cancer therapy. Curr. Med. Chem 2012, 19, 3230–3240. [Google Scholar]
- Wang, H.M.; Soica, C.; Wenz, G. A comparison investigation on the solubilization of betulin and betulinic acid in cyclodextrin derivatives. Nat. Prod. Commun 2012, 7, 289–291. [Google Scholar]
- Yamamura, S.; Momose, Y. Quantitative analysis of crystalline pharmaceuticals in powders and tablets by a pattern-fitting procedure using X-ray powder diffraction data. Int. J. Pharm 2001, 212, 203–212. [Google Scholar]
- Dehelean, C.A.; Feflea, S.; Molnár, J.; Zupko, I.; Soica, C. Betulin as an antitumor agent tested in vitro on A431, HeLa and MCF7, and as an angiogenic inhibitor in vivo in the CAM assay. Nat. Prod. Commun 2012, 7, 981–985. [Google Scholar]
- Li, Y.; He, K.; Huang, Y.; Zheng, D.; Gao, C.; Cui, L.; Jin, Y.H. Betulin induces mitochondrial cytochrome c release associated apoptosis in human cancer cells. Mol. Carcinog 2010, 49, 630–640. [Google Scholar]
- Rzeski, W.; Stepulak, A.; Szymański, M.; Juszczak, M.; Grabarska, A.; Sifringer, M.; Kaczor, J.; Kandefer-Szerszeń, M. Betulin elicits anti-cancer effects in tumour primary cultures and cell lines in vitro. Basic Clin. Pharmacol. Toxicol 2009, 105, 425–432. [Google Scholar]
- Eiznhamer, D.A.; Xu, Z.-Q. Betulinic acid: A promising anticancer candidate. IDrugs 2004, 7, 359–373. [Google Scholar]
- Fulda, S.; Debatin, K.-M. Sensitization for anticancer drug-induced apoptosis by betulinic acid. Neoplasia 2005, 7, 162–170. [Google Scholar]
- Sami, A.; Taru, M.; Salme, K.; Jari, Y-K. Pharmacological properties of the ubiquitous natural product betulin. Eur. J. Pharm. Sci 2006, 29, 1–13. [Google Scholar]
- Mendonca, E.A.; Lira, M.C.; Rabello, M.M.; Cavalcanti, I.M.; Galdino, S.L.; Pitta, I.R.; Lima Mdo, C.; Pitta, M.G.; Hernandes, M.Z.; Santos-Magalhães, N.S. Enhanced antiproliferative activity of the new anticancer candidate LPSF/AC04 in cyclodextrin inclusion complexes encapsulated into liposomes. AAPS PharmSciTech 2012, 13, 1355–1366. [Google Scholar]
- Pourgholami, M.H.; Wangoo, K.T.; Morris, D.L. Albendazole-cyclodextrin complex: Enhanced cytotoxicity in ovarian cancer cells. Anticancer Res 2008, 28, 2775–2779. [Google Scholar]
- Liu, Y.; Chen, G.S.; Chen, Y.; Cao, D.X.; Ge, Z.Q.; Yuan, Y.J. Inclusion complexes of paclitaxel and oligo(ethylenediamino) bridged bis(b-cyclodextrin)s: Solubilization and antitumor activity. Bioorg. Med. Chem 2004, 12, 5767–5775. [Google Scholar]
- Rieber, M.; Rieber Strasberg, M. Signalling responses linked to betulinic acid-induced apoptosis are antagonized by MEK inhibitor U0126 in adherent or 3D spheroid melanoma irrespective of p53 status. Int. J. Cancer 2006, 118, 1135–1143. [Google Scholar]
- Cheng, Y.Q.; Chen, Y.; Wu, Q.L.; Fang, J.; Yang, L.J. Effect of betulinic acid on inducing apoptosis of human multiple myeloma cell line RPMI-8226. Zhongguo Shi Yan Xue Ye Xue Za Zhi 2009, 17, 1224–1229. [Google Scholar]
- Chen, Z.; Wu, Q.; Chen, Y.; He, J. Effects of betulinic acid on proliferation and apoptosis in Jurkat cells and its in vitro mechanism. J. Hua Zhong Univ. Sci. Technol. Med. Sci 2008, 28, 634–638. [Google Scholar]
- Vadivelu, R.K.; Yeap, S.K.; Ali, A.M.; Hamid, M.; Alitheen, N.B. Betulinic acid inhibits growth of cultured vascular smooth muscle cells arrest and apoptosis. Evid-Based Complement. Altern. Med 2012, 2012, 251362. [Google Scholar]
- Fulda, S.; Kroemer, G. Targeting mitochondrial apoptosis by betulinic acid in human cancers. Drug Discov. Today 2009, 14, 885–890. [Google Scholar]
- Santos, R.C.; Salvador, J.A.R.; Cortés, R.; Pachón, G.; Marín, S.; Cascante, M. New betulinic acid derivatives induce potent and selective antiproliferative activity through cell cycle arrest at the S phase and caspase dependent apoptosis in human cancer cells. Biochimie 2011, 93, 1065–1075. [Google Scholar]
- Zaritskaya, L.; Shurin, M.R.; Sayers, T.J.; Malyguine, A.M. New flow cytometric assays for monitoring cell-mediated cytotoxicity. Expert Rev. Vaccines 2010, 9, 601–616. [Google Scholar]
- Mullauer, F.B.; Kessler, J.H.; Medema, J.P. Betulinic acid induces cytochrome c release and apoptosis in a Bax/Bak-independent, permeability transition pore dependent fashion. Apoptosis 2009, 14, 191–202. [Google Scholar]
- Fulda, S.; Debatin, K.M. Betulinic acid induces apoptosis through a direct effect on mitochondria in neuroectodermal tumors. Med. Pediatr. Oncol 2000, 35, 616–618. [Google Scholar]
- Liu, W-K.; Ho, J.C.K.; Cheung, F.W.K.; Liu, B.P.L.; Ye, W-C.; Che, C-T. Apoptotic activity of betulinic acid derivatives on murine melanoma B16 cell line. Eur. J. Pharmacol 2004, 498, 71–78. [Google Scholar]
- Serradell, M.; Díaz-Ricart, M.; Cases, A.; Petriz, J.; Ordinas, A.; Escolar, G. Uraemic medium accelerates proliferation but does not induce apoptosis of endothelial cells in culture. Nephrol. Dial. Transplant 2003, 18, 1079–1085. [Google Scholar]
- Rubio, N.; Torres, C. Interferon-γ induces proliferation but not apoptosis in murine astrocytes through the differential expression of the myc proto-oncogene family. Mol. Brain Res 1999, 71, 104–110. [Google Scholar]
- Harmand, P.O.; Duval, R.; Delage, C.; Simon, A. Ursolic acid induces apoptosis through mitochondrial intrinsic pathway and caspase-3 activation in M4Beu melanoma cells. Int. J. Cancer 2005, 114, 1–11. [Google Scholar]
- Danciu, C.; Borcan, F.; Bojin, F.; Zupko, I.; Dehelean, C. Effect of the isoflavone genistein on tumor size, metastasis potential and melanization in a B16 mouse model of murine melanoma. Nat. Prod. Commun 2013, 8, 343–346. [Google Scholar]
- Cinta Pinzaru, S.; Falamas, A.; Dehelean, C.A. Molecular conformation changes along the malignancy revealed by optical nanosensors. J. Cell. Mol. Med 2013, 17, 277–286. [Google Scholar]
- Dehelean, C.A.; Feflea, S.; Ganta, S.; Amiji, M.M. Anti-angiogenic effects of betulinic acid administered in nanoemulsion formulation using chorioallantoic membrane assay. J. Biomed. Nanotechnol 2011, 7, 317–324. [Google Scholar]
- Galgon, T.; Wohlrab, W.; Dräger, B. Betulinic acid induces apoptosis in skin cancer cells and differentiation in normal human keratinocytes. Exp. Dermatol 2005, 14, 736–743. [Google Scholar]
- Ciurlea, S.A.; Dehelean, C.A.; Ionescu, D.; Berko, S.; Csanyi, E.; Hadaruga, D.I.; Ganta, S.; Amiji, M.M. A comparative study regarding melanoma activity of betulinic acid on topical ointment vs. systemic nanoemulsion delivery systems. J. Agroaliment. Process. Technol 2010, 16, 420–426. [Google Scholar]
- Fulda, S. Betulinic acid for cancer treatment and prevention. Int. J. Mol. Sci 2008, 9, 1096–1107. [Google Scholar]
- Yogeeswari, P.; Sriram, D. Betulinic acid and its derivatives: A review on their biological properties. Curr. Med. Chem 2005, 12, 657–666. [Google Scholar]
- Mertens-Talcott, S.U.; Noratto, G.D.; Li, X.; Angel-Morales, G.; Bertoldi, M.C.; Safe, S. Betulinic acid decreases ER-negative breast cancer cell growth in vitro and in vivo: Role of Sp transcription factors and microRNA-27a: ZBTB10. Mol. Carcinog 2013, 52, 591–602. [Google Scholar]
- Şoica, C.; Dehelean, C.; Danciu, C.; Wang, H.M.; Wenz, G.; Ambrus, R.; Bojin, F.; Anghel, M. Betulin complex in γ-cyclodextrin derivatives: Properties and antineoplasic activities in vitro and in vivo tumor models. Int. J. Mol. Sci 2012, 13, 14992–15011. [Google Scholar]
- Xu, W.; Ling, P.; Zhang, T. Polymeric micelles, a promising drug delivery system to enhance bioavailability of poorly water-soluble drugs. J. Drug Deliv 2013, 2013, 340315. [Google Scholar]
- Lee, S.C.; Huh, K.M.; Lee, J.; Cho, Y.W.; Galinsky, R.E.; Park, K. Hydrotropic polymeric micelles for enhanced paclitaxel solubility: In vitro and in vivo characterization. Biomacromolecules 2007, 8, 202–208. [Google Scholar]
- Awasthi, N.; Zhang, C.; Schwarz, A.M.; Hinz, S.; Wang, C.; Williams, N.S.; Schwarz, M.A.; Schwarz, R.E. Comparative benefits of Nab-paclitaxel over gemcitabine or polysorbate-based docetaxel in experimental pancreatic cancer. Carcinogenesis 2013, 34, 2361–2369. [Google Scholar]
- Giavazzi, R.; Campbell, D.E.; Jessup, J.M.; Cleary, K.; Fidler, I.J. Metastatic behavior of tumor cells isolated from primary and metastatic human colorectal carcinomas implanted into different sites in nude mice. Cancer Res 1986, 46, 1928–1933. [Google Scholar]
- Hoshino, T.; Matsuda, M.; Yamashita, Y.; Takehara, M.; Fukuya, M.; Mineda, K.; Maji, D.; Ihn, H.; Adachi, H.; Sobue, G.; et al. Suppression of melanin production by expression of hsp70. J. Biol. Chem 2010, 285, 13254–13263. [Google Scholar]










| Sample (10 mM) | Cell cycle phases for non-metastatic B164A5 cells | Cell cycle phases for metastatic B164A5 cells | ||||||
|---|---|---|---|---|---|---|---|---|
| under-G0 (%) | G0/G1 (%) | S (%) | G2/M (%) | under-G0 (%) | G0/G1 (%) | S (%) | G2/M (%) | |
| Control | 2.88 ± 0.14 | 64.32 ± 2.13 | 21.17 ± 0.22 | 11.63 ± 0.17 | 1.24 ± 1.28 | 71.68 ± 3.14 | 18.43 ± 1.19 | 8.65 ± 1.21 |
| DMSO | 1.49 ± 0.22 | 69.43 ± 1.39 | 19.81 ± 0.34 | 9.27 ± 0.31 | 1.93 ± 1.17 | 68.39 ± 2.67 | 17.03 ± 0.28 | 12.65 ± 1.19 |
| BA | 0.79 ± 3.4 | 87.61 ± 1.64 | 6.14 ± 0.28 | 5.46 ± 0.25 | 1.39 ± 2.21 | 87.09 ± 3.03 | 7.45 ± 1.65 | 4.07 ± 3.42 |
| BA:GCDG | 2.29 ± 1.27 | 79.87 ± 1.94 | 11.98 ± 1.73 | 5.86 ± 2.01 | 2.97 ± 2.27 | 77.53 ± 2.29 | 8.29 ± 3.02 | 11.21 ± 1.98 |
© 2014 by the authors; licensee MDPI, Basel, Switzerland This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Soica, C.; Danciu, C.; Savoiu-Balint, G.; Borcan, F.; Ambrus, R.; Zupko, I.; Bojin, F.; Coricovac, D.; Ciurlea, S.; Avram, S.; et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. Int. J. Mol. Sci. 2014, 15, 8235-8255. https://doi.org/10.3390/ijms15058235
Soica C, Danciu C, Savoiu-Balint G, Borcan F, Ambrus R, Zupko I, Bojin F, Coricovac D, Ciurlea S, Avram S, et al. Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells. International Journal of Molecular Sciences. 2014; 15(5):8235-8255. https://doi.org/10.3390/ijms15058235
Chicago/Turabian StyleSoica, Codruta, Corina Danciu, Germaine Savoiu-Balint, Florin Borcan, Rita Ambrus, Istvan Zupko, Florina Bojin, Dorina Coricovac, Sorina Ciurlea, Stefana Avram, and et al. 2014. "Betulinic Acid in Complex with a Gamma-Cyclodextrin Derivative Decreases Proliferation and in Vivo Tumor Development of Non-Metastatic and Metastatic B164A5 Cells" International Journal of Molecular Sciences 15, no. 5: 8235-8255. https://doi.org/10.3390/ijms15058235





