BMP-Functionalised Coatings to Promote Osteogenesis for Orthopaedic Implants
Abstract
:1. Introduction
2. BMP (Bone Morphogenetic Protein)
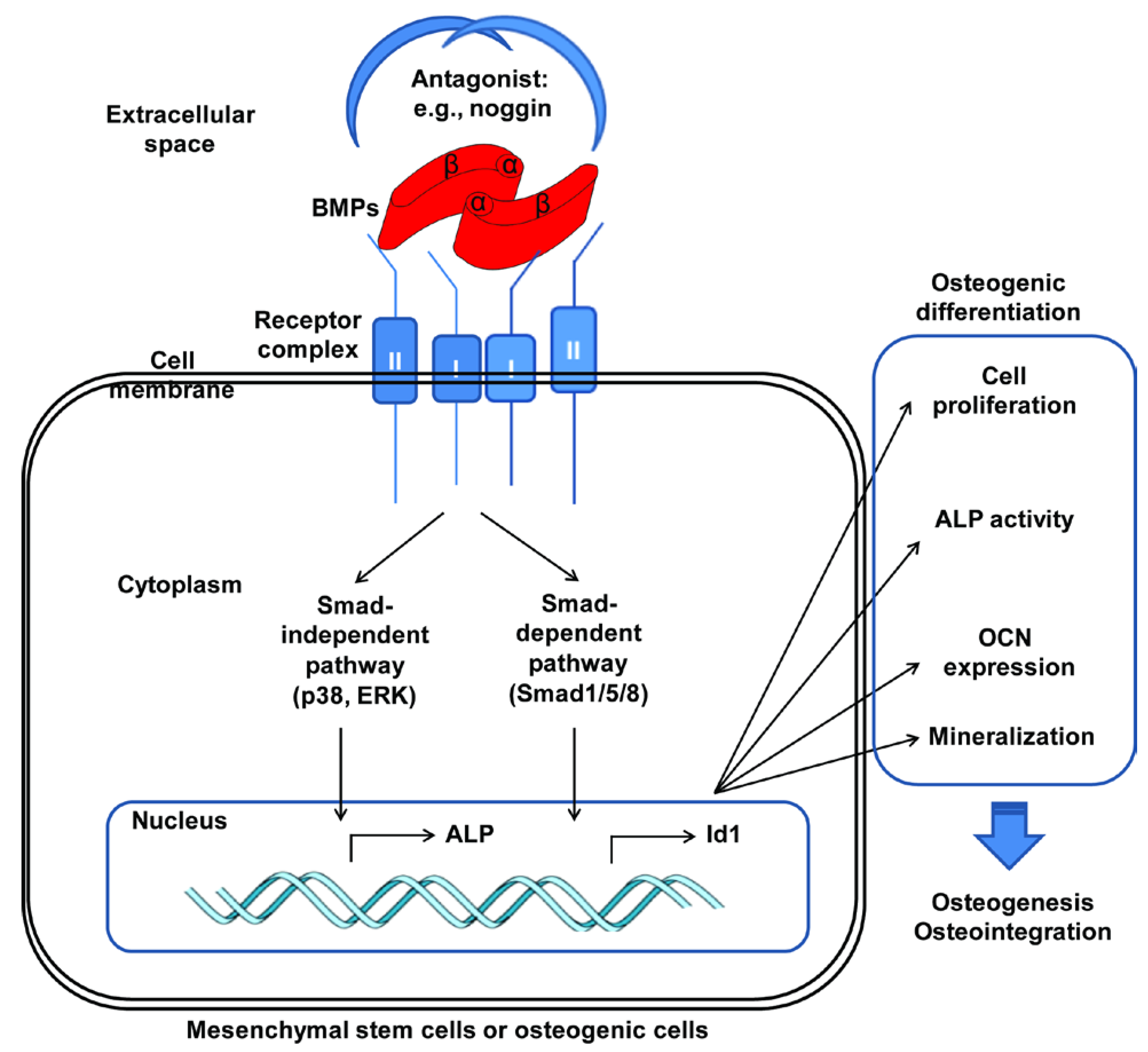
3. Adsorption of BMPs
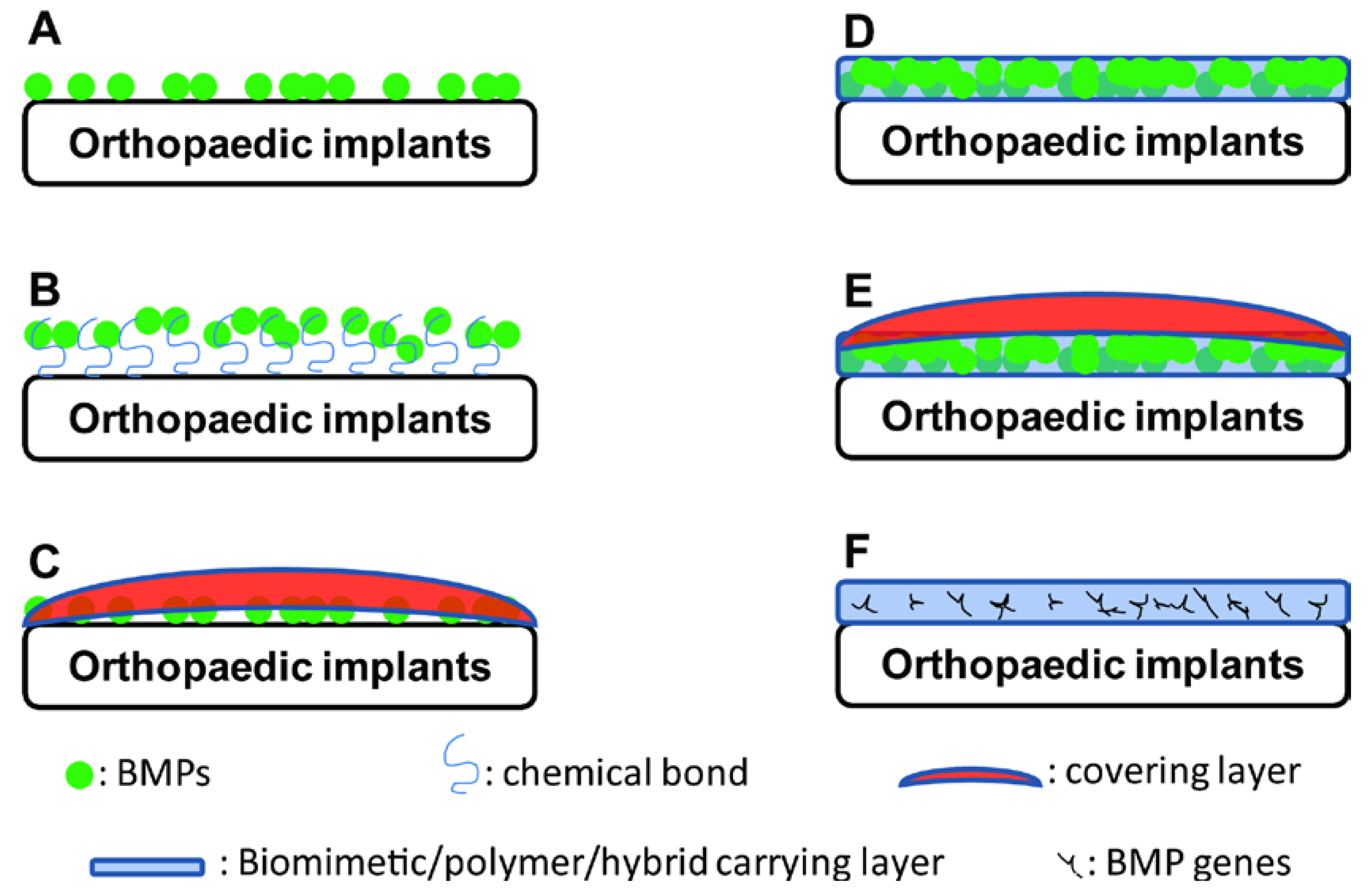
4. BMP-Carrying Coatings
5. BMP-Functionalised Biomimetic Coatings
6. Coatings for the Delivery of BMP Genes
6.1. Coatings with Non-Viral Vectors Delivering BMP Genes
6.2. Coatings with Viral Vectors Delivering BMP Genes
7. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Claffey, N.; Clarke, E.; Polyzois, I.; Renvert, S. Surgical treatment of peri-implantitis. J. Clin. Periodontol. 2008, 35, 316–332. [Google Scholar] [CrossRef]
- Saito, N.; Takaoka, K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 2003, 24, 2287–2293. [Google Scholar] [CrossRef]
- Kretlow, J.D.; Mikos, A.G. Review: Mineralization of synthetic polymer scaffolds for bone tissue engineering. Tissue Eng. 2007, 13, 927–938. [Google Scholar] [CrossRef]
- Silber, J.S.; Anderson, D.G.; Daffner, S.D.; Brislin, B.T.; Leland, J.M.; Hilibrand, A.S.; Vaccaro, A.R.; Albert, T.J. Donor site morbidity after anterior iliac crest bone harvest for single-level anterior cervical discectomy and fusion. Spine 2003, 28, 134–139. [Google Scholar] [CrossRef]
- Heary, R.F.; Schlenk, R.P.; Sacchieri, T.A.; Barone, D.; Brotea, C. Persistent iliac crest donor site pain: Independent outcome assessment. Neurosurgery 2002, 50, 510–516. [Google Scholar]
- Orthopedic Implants. Available online: http://orthopedicimplants.wordpress.com/ (assessed on 5 May 2014).
- Mantripragada, V.P.; Lecka-Czernik, B.; Ebraheim, N.A.; Jayasuriya, A.C. An overview of recent advances in designing orthopedic and craniofacial implants. J. Biomed. Mater. Res. A 2013, 101, 3349–3364. [Google Scholar]
- Carson, J.S.; Bostrom, M.P. Synthetic bone scaffolds and fracture repair. Injury 2007, 38, S33–S37. [Google Scholar] [CrossRef]
- Sokolsky-Papkov, M.; Agashi, K.; Olaye, A.; Shakesheff, K.; Domb, A.J. Polymer carriers for drug delivery in tissue engineering. Adv. Drug Deliv. Rev. 2007, 59, 187–206. [Google Scholar] [CrossRef]
- Bessa, P.C.; Casal, M.; Reis, R.L. Bone morphogenetic proteins in tissue engineering: The road from the laboratory to the clinic, part I (basic concepts). J. Tissue Eng. Regen. Med. 2008, 2, 1–13. [Google Scholar]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part A: Current challenges in BMP delivery. Biotechnol. Lett. 2009, 31, 1817–1824. [Google Scholar] [CrossRef]
- Haidar, Z.S.; Hamdy, R.C.; Tabrizian, M. Delivery of recombinant bone morphogenetic proteins for bone regeneration and repair. Part B: Delivery systems for BMPs in orthopaedic and craniofacial tissue engineering. Biotechnol. Lett. 2009, 31, 1825–1835. [Google Scholar] [CrossRef]
- Shields, L.B.; Raque, G.H.; Glassman, S.D.; Campbell, M.; Vitaz, T.; Harpring, J.; Shields, C.B. Adverse effects associated with high-dose recombinant human bone morphogenetic protein-2 use in anterior cervical spine fusion. Spine 2006, 31, 542–547. [Google Scholar] [CrossRef]
- Smith, D.M.; Cooper, G.M.; Mooney, M.P.; Marra, K.G.; Losee, J.E. Bone morphogenetic protein 2 therapy for craniofacial surgery. J. Craniofac. Surg. 2008, 19, 1244–1259. [Google Scholar] [CrossRef]
- Toth, J.M.; Boden, S.D.; Burkus, J.K.; Badura, J.M.; Peckham, S.M.; McKay, W.F. Short-term osteoclastic activity induced by locally high concentrations of recombinant human bone morphogenetic protein-2 in a cancellous bone environment. Spine 2009, 34, 539–550. [Google Scholar] [CrossRef]
- Lutolf, M.P.; Weber, F.E.; Schmoekel, H.G.; Schense, J.C.; Kohler, T.; Muller, R.; Hubbell, J.A. Repair of bone defects using synthetic mimetics of collagenous extracellular matrices. Nat. Biotechnol. 2003, 21, 513–518. [Google Scholar] [CrossRef]
- Ducy, P.; Karsenty, G. The family of bone morphogenetic proteins. Kidney Int. 2000, 57, 2207–2214. [Google Scholar] [CrossRef]
- Urist, M.R. Bone: Formation by autoinduction. Science 1965, 150, 893–899. [Google Scholar]
- Wang, E.A.; Rosen, V.; Cordes, P.; Hewick, R.M.; Kriz, M.J.; Luxenberg, D.P.; Sibley, B.S.; Wozney, J.M. Purification and characterization of other distinct bone-inducing factors. Proc. Natl. Acad. Sci. USA 1988, 85, 9484–9488. [Google Scholar] [CrossRef]
- Reddi, A.H.; Reddi, A. Bone morphogenetic proteins (BMPs): From morphogens to metabologens. Cytokine Growth Factor Rev. 2009, 20, 341–342. [Google Scholar] [CrossRef]
- Reddi, A.H. BMPs: From bone morphogenetic proteins to body morphogenetic proteins. Cytokine Growth Factor Rev. 2005, 16, 249–250. [Google Scholar] [CrossRef]
- Liao, W.X.; Moore, R.K.; Otsuka, F.; Shimasaki, S. Effect of intracellular interactions on the processing and secretion of bone morphogenetic protein-15 (BMP-15) and growth and differentiation factor-9. Implication of the aberrant ovarian phenotype of BMP-15 mutant sheep. J. Biol. Chem. 2003, 278, 3713–3719. [Google Scholar]
- Sieber, C.; Kopf, J.; Hiepen, C.; Knaus, P. Recent advances in BMP receptor signaling. Cytokine Growth Factor Rev. 2009, 20, 343–355. [Google Scholar] [CrossRef]
- Levi, B.; Hyun, J.S.; Nelson, E.R.; Li, S.; Montoro, D.T.; Wan, D.C.; Jia, F.J.; Glotzbach, J.C.; James, A.W.; Lee, M.; et al. Nonintegrating knockdown and customized scaffold design enhances human adipose-derived stem cells in skeletal repair. Stem Cells 2011, 29, 2018–2029. [Google Scholar]
- Tseng, Y.H.; Kokkotou, E.; Schulz, T.J.; Huang, T.L.; Winnay, J.N.; Taniguchi, C.M.; Tran, T.T.; Suzuki, R.; Espinoza, D.O.; Yamamoto, Y.; et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature 2008, 454, 1000–1004. [Google Scholar]
- Kim, H.J.; Im, G.I. Combination of transforming growth factor-beta2 and bone morphogenetic protein 7 enhances chondrogenesis from adipose tissue-derived mesenchymal stem cells. Tissue Eng. A 2009, 15, 1543–1551. [Google Scholar] [CrossRef]
- Derynck, R.; Zhang, Y.E. Smad-dependent and Smad-independent pathways in TGF-beta family signalling. Nature 2003, 425, 577–584. [Google Scholar] [CrossRef]
- Guo, J.; Wu, G. The signaling and functions of heterodimeric bone morphogenetic proteins. Cytokine Growth Factor Rev. 2012, 23, 61–67. [Google Scholar] [CrossRef]
- Miyazono, K.; Kamiya, Y.; Morikawa, M. Bone morphogenetic protein receptors and signal transduction. J. Biochem. 2010, 147, 35–51. [Google Scholar] [CrossRef]
- Xiao, G.; Gopalakrishnan, R.; Jiang, D.; Reith, E.; Benson, M.D.; Franceschi, R.T. Bone morphogenetic proteins, extracellular matrix, and mitogen-activated protein kinase signaling pathways are required for osteoblast-specific gene expression and differentiation in MC3T3-E1 cells. J. Bone Miner. Res. 2002, 17, 101–110. [Google Scholar] [CrossRef]
- Takada, T.; Katagiri, T.; Ifuku, M.; Morimura, N.; Kobayashi, M.; Hasegawa, K.; Ogamo, A.; Kamijo, R. Sulfated polysaccharides enhance the biological activities of bone morphogenetic proteins. J. Biol. Chem. 2003, 278, 43229–43235. [Google Scholar] [CrossRef]
- Osses, N.; Gutierrez, J.; Lopez-Rovira, T.; Ventura, F.; Brandan, E. Sulfation is required for bone morphogenetic protein 2-dependent Id1 induction. Biochem. Biophs. Res. Commun. 2006, 344, 1207–1215. [Google Scholar] [CrossRef]
- Zhao, B.; Katagiri, T.; Toyoda, H.; Takada, T.; Yanai, T.; Fukuda, T.; Chung, U.I.; Koike, T.; Takaoka, K.; Kamijo, R. Heparin potentiates the in vivo ectopic bone formation induced by bone morphogenetic protein-2. J. Biol. Chem. 2006, 281, 23246–23253. [Google Scholar] [CrossRef]
- Zhou, H.; Qian, J.; Wang, J.; Yao, W.; Liu, C.; Chen, J.; Cao, X. Enhanced bioactivity of bone morphogenetic protein-2 with low dose of 2-N, 6-O-sulfated chitosan in vitro and in vivo. Biomaterials 2009, 30, 1715–1724. [Google Scholar] [CrossRef]
- Zheng, Y.; Wu, G.; Zhao, J.; Wang, L.; Sun, P.; Gu, Z. rhBMP2/7 heterodimer: An osteoblastogenesis inducer of not higher potency but lower effective concentration compared with rhBMP2 and rhBMP7 homodimers. Tissue Eng. A 2010, 16, 879–887. [Google Scholar]
- Zhu, W.; Kim, J.; Cheng, C.; Rawlins, B.A.; Boachie-Adjei, O.; Crystal, R.G.; Hidaka, C. Noggin regulation of bone morphogenetic protein (BMP) 2/7 heterodimer activity in vitro. Bone 2006, 39, 61–71. [Google Scholar] [CrossRef]
- Wang, J.; Zheng, Y.; Zhao, J.; Liu, T.; Gao, L.; Gu, Z.; Wu, G. Low-dose rhBMP2/7 heterodimer to reconstruct peri-implant bone defects: A micro-CT evaluation. J. Clin. Periodontol. 2012, 39, 98–105. [Google Scholar] [CrossRef]
- Luo, J.; Tang, M.; Huang, J.; He, B.C.; Gao, J.L.; Chen, L.; Zuo, G.W.; Zhang, W.; Luo, Q.; Shi, Q.; et al. TGFbeta/BMP type I receptors ALK1 and ALK2 are essential for BMP9-induced osteogenic signaling in mesenchymal stem cells. J. Biol. Chem. 2010, 285, 29588–29598. [Google Scholar] [CrossRef]
- Wang, Y.; Hong, S.; Li, M.; Zhang, J.; Bi, Y.; He, Y.; Liu, X.; Nan, G.; Su, Y.; Zhu, G.; et al. Noggin resistance contributes to the potent osteogenic capability of BMP9 in mesenchymal stem cells. J. Orthop. Res. 2013, 31, 1796–1803. [Google Scholar]
- Carragee, E.J.; Hurwitz, E.L.; Weiner, B.K. A critical review of recombinant human bone morphogenetic protein-2 trials in spinal surgery: Emerging safety concerns and lessons learned. Spine J. 2011, 11, 471–491. [Google Scholar] [CrossRef]
- Park, D.K.; Kim, S.S.; Thakur, N.; Boden, S.D. Use of recombinant human bone morphogenetic protein-2 with local bone graft instead of iliac crest bone graft in posterolateral lumbar spine arthrodesis. Spine 2013, 38, E738–E747. [Google Scholar] [CrossRef]
- Huh, J.B.; Park, C.K.; Kim, S.E.; Shim, K.M.; Choi, K.H.; Kim, S.J.; Shim, J.S.; Shin, S.W. Alveolar ridge augmentation using anodized implants coated with Escherichia coli-derived recombinant human bone morphogenetic protein 2. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endod. 2011, 112, 42–49. [Google Scholar] [CrossRef]
- Shiels, S.M.; Solomon, K.D.; Pilia, M.; Appleford, M.R.; Ong, J.L. BMP-2 tethered hydroxyapatite for bone tissue regeneration: Coating chemistry and osteoblast attachment. J. Biomed. Mater. Res. A 2012, 100, 3117–3123. [Google Scholar]
- Shiels, S.; Oh, S.; Bae, C.; Guda, T.; Singleton, B.; Dean, D.D.; Wenke, J.C.; Appleford, M.R.; Ong, J.L. Evaluation of BMP-2 tethered polyelectrolyte coatings on hydroxyapatite scaffolds in vivo. J. Biomed. Mater. Res. B 2012, 100, 1782–1791. [Google Scholar]
- Kloss, F.R.; Gassner, R.; Preiner, J.; Ebner, A.; Larsson, K.; Hachl, O.; Tuli, T.; Rasse, M.; Moser, D.; Laimer, K.; et al. The role of oxygen termination of nanocrystalline diamond on immobilisation of BMP-2 and subsequent bone formation. Biomaterials 2008, 29, 2433–2442. [Google Scholar] [CrossRef]
- La, W.G.; Park, S.; Yoon, H.H.; Jeong, G.J.; Lee, T.J.; Bhang, S.H.; Han, J.Y.; Char, K.; Kim, B.S. Delivery of a therapeutic protein for bone regeneration from a substrate coated with graphene oxide. Small 2013, 9, 4051–4060. [Google Scholar] [CrossRef]
- Ko, E.; Yang, K.; Shin, J.; Cho, S.W. Polydopamine-assisted osteoinductive peptide immobilization of polymer scaffolds for enhanced bone regeneration by human adipose-derived stem cells. Biomacromolecules 2013, 14, 3202–3213. [Google Scholar] [CrossRef]
- Chien, C.Y.; Tsai, W.B. Poly(dopamine)-assisted immobilization of Arg-Gly-Asp peptides, hydroxyapatite, and bone morphogenic protein-2 on titanium to improve the osteogenesis of bone marrow stem cells. ACS Appl. Mater. Interfaces 2013, 5, 6975–6983. [Google Scholar] [CrossRef]
- Kim, M.; Jung, W.K.; Kim, G. Bio-composites composed of a solid free-form fabricated polycaprolactone and alginate-releasing bone morphogenic protein and bone formation peptide for bone tissue regeneration. Bioprocess Biosyst. Eng. 2013, 36, 1725–1734. [Google Scholar] [CrossRef]
- Peterson, A.M.; Mohwald, H.; Shchukin, D.G. pH-controlled release of proteins from polyelectrolyte-modified anodized titanium surfaces for implant applications. Biomacromolecules 2012, 13, 3120–3126. [Google Scholar] [CrossRef]
- Ruhe, P.Q.; Boerman, O.C.; Russel, F.G.; Spauwen, P.H.; Mikos, A.G.; Jansen, J.A. Controlled release of rhBMP-2 loaded poly(dl-lactic-co-glycolic acid)/calcium phosphate cement composites in vivo. J. Control. Release 2005, 106, 162–171. [Google Scholar] [CrossRef]
- Ginebra, M.P.; Traykova, T.; Planell, J.A. Calcium phosphate cements as bone drug delivery systems: A review. J. Control. Release 2006, 113, 102–110. [Google Scholar] [CrossRef]
- Ruhe, P.Q.; Kroese-Deutman, H.C.; Wolke, J.G.; Spauwen, P.H.; Jansen, J.A. Bone inductive properties of rhBMP-2 loaded porous calcium phosphate cement implants in cranial defects in rabbits. Biomaterials 2004, 25, 2123–2132. [Google Scholar] [CrossRef]
- Zhao, J.; Shen, G.; Liu, C.; Wang, S.; Zhang, W.; Zhang, X.; Ye, D.; Wei, J.; Zhang, Z.; Jiang, X. Enhanced healing of rat calvarial defects with sulfated chitosan-coated calcium-deficient hydroxyapatite/bone morphogenetic protein 2 scaffolds. Tissue Eng. A 2012, 18, 185–197. [Google Scholar] [CrossRef]
- Schmidmaier, G.; Wildemann, B.; Stemberger, A.; Haas, N.P.; Raschke, M. Biodegradable poly(D,L-lactide) coating of implants for continuous release of growth factors. J. Biomed. Mater. Res. 2001, 58, 449–455. [Google Scholar] [CrossRef]
- Strobel, C.; Bormann, N.; Kadow-Romacker, A.; Schmidmaier, G.; Wildemann, B. Sequential release kinetics of two (gentamicin and BMP-2) or three (gentamicin, IGF-I and BMP-2) substances from a one-component polymeric coating on implants. J. Control. Release 2011, 156, 37–45. [Google Scholar] [CrossRef]
- Kang, S.W.; Kim, J.S.; Park, K.S.; Cha, B.H.; Shim, J.H.; Kim, J.Y.; Cho, D.W.; Rhie, J.W.; Lee, S.H. Surface modification with fibrin/hyaluronic acid hydrogel on solid-free form-based scaffolds followed by BMP-2 loading to enhance bone regeneration. Bone 2011, 48, 298–306. [Google Scholar] [CrossRef]
- Zhang, Q.; Tan, K.; Zhang, Y.; Ye, Z.; Tan, W.S.; Lang, M. In situ controlled release of rhBMP-2 in gelatin-coated 3D porous poly(epsilon-caprolactone) scaffolds for homogeneous bone tissue formation. Biomacromolecules 2014, 15, 84–94. [Google Scholar] [CrossRef]
- Jun, S.H.; Lee, E.J.; Jang, T.S.; Kim, H.E.; Jang, J.H.; Koh, Y.H. Bone morphogenic protein-2 (BMP-2) loaded hybrid coating on porous hydroxyapatite scaffolds for bone tissue engineering. J. Mater. Sci. Mater. Med. 2013, 24, 773–782. [Google Scholar]
- Macdonald, M.L.; Rodriguez, N.M.; Shah, N.J.; Hammond, P.T. Characterization of tunable FGF-2 releasing polyelectrolyte multilayers. Biomacromolecules 2010, 11, 2053–2059. [Google Scholar] [CrossRef]
- Wood, K.C.; Chuang, H.F.; Batten, R.D.; Lynn, D.M.; Hammond, P.T. Controlling interlayer diffusion to achieve sustained, multiagent delivery from layer-by-layer thin films. Proc. Natl. Acad. Sci. USA 2006, 103, 10207–10212. [Google Scholar] [CrossRef]
- Macdonald, M.L.; Samuel, R.E.; Shah, N.J.; Padera, R.F.; Beben, Y.M.; Hammond, P.T. Tissue integration of growth factor-eluting layer-by-layer polyelectrolyte multilayer coated implants. Biomaterials 2011, 32, 1446–1453. [Google Scholar] [CrossRef]
- Guillot, R.; Gilde, F.; Becquart, P.; Sailhan, F.; Lapeyrere, A.; Logeart-Avramoglou, D.; Picart, C. The stability of BMP loaded polyelectrolyte multilayer coatings on titanium. Biomaterials 2013, 34, 5737–5746. [Google Scholar] [CrossRef]
- Van den Beucken, J.J.; Walboomers, X.F.; Boerman, O.C.; Vos, M.R.; Sommerdijk, N.A.; Hayakawa, T.; Fukushima, T.; Okahata, Y.; Nolte, R.J.; Jansen, J.A. Functionalization of multilayered DNA-coatings with bone morphogenetic protein 2. J. Control. Release 2006, 113, 63–72. [Google Scholar] [CrossRef]
- Kokubo, T.; Ito, S.; Huang, Z.T.; Hayashi, T.; Sakka, S.; Kitsugi, T.; Yamamuro, T. Ca, P-rich layer formed on high-strength bioactive glass-ceramic A-W. J. Biomed. Mater. Res. 1990, 24, 331–343. [Google Scholar] [CrossRef]
- Ohgushi, H.; Caplan, A.I. Stem cell technology and bioceramics: From cell to gene engineering. J. Biomed. Mater. Res. 1999, 48, 913–927. [Google Scholar] [CrossRef]
- Barrere, F.; van der Valk, C.M.; Meijer, G.; Dalmeijer, R.A.; de Groot, K.; Layrolle, P. Osteointegration of biomimetic apatite coating applied onto dense and porous metal implants in femurs of goats. J. Biomed. Mater. Res. B 2003, 67, 655–665. [Google Scholar]
- Li, P. Biomimetic nano-apatite coating capable of promoting bone ingrowth. J. Biomed. Mater. Res. A 2003, 66, 79–85. [Google Scholar]
- Yan, W.Q.; Nakamura, T.; Kawanabe, K.; Nishigochi, S.; Oka, M.; Kokubo, T. Apatite layer-coated titanium for use as bone bonding implants. Biomaterials 1997, 18, 1185–1190. [Google Scholar] [CrossRef]
- Nagano, M.; Kitsugi, T.; Nakamura, T.; Kokubo, T.; Tanahashi, M. Bone bonding ability of an apatite-coated polymer produced using a biomimetic method: A mechanical and histological study in vivo. J. Biomed. Mater. Res. 1996, 31, 487–494. [Google Scholar] [CrossRef]
- Tanahashi, M.; Matsuda, T. Surface functional group dependence on apatite formation on self-assembled monolayers in a simulated body fluid. J. Biomed. Mater. Res. 1997, 34, 305–315. [Google Scholar] [CrossRef]
- Filmon, R.; Grizon, F.; Basle, M.F.; Chappaard, D. Effects of negatively charged groups (carboxymethyl) on the calcification of poly(2-hydroxyethyl methacrylate). Biomaterials 2002, 23, 3053–3059. [Google Scholar] [CrossRef]
- Liu, Y.; Layrolle, P.; de Bruijn, J.; van Blitterswijk, C.; de Groot, K. Biomimetic coprecipitation of calcium phosphate and bovine serum albumin on titanium alloy. J. Biomed. Mater. Res. 2001, 57, 327–335. [Google Scholar] [CrossRef]
- Liu, Y.; Huse, R.O.; de Groot, K.; Buser, D.; Hunziker, E.B. Delivery mode and efficacy of BMP-2 in association with implants. J. Dent. Res. 2007, 86, 84–89. [Google Scholar] [CrossRef]
- Liu, Y.; Hunziker, E.B.; Layrolle, P.; van Blitterswijk, C.; Calvert, P.D.; de Groot, K. Remineralization of demineralized albumin-calcium phosphate coatings. J. Biomed. Mater. Res. A 2003, 67, 1155–1162. [Google Scholar]
- Wu, G.; Hunziker, E.B.; Zheng, Y.; Wismeijer, D.; Liu, Y. Functionalization of deproteinized bovine bone with a coating-incorporated depot of BMP-2 renders the material efficiently osteoinductive and suppresses foreign-body reactivity. Bone 2011, 49, 1323–1330. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. Biomimetic coating of organic polymers with a protein-functionalized layer of calcium phosphate: The surface properties of the carrier influence neither the coating characteristics nor the incorporation mechanism or release kinetics of the protein. Tissue Eng. C 2010, 16, 1255–1265. [Google Scholar] [CrossRef]
- Barrere, F.; van, B.C.; de, G.K.; Layrolle, P. Nucleation of biomimetic Ca-P coatings on ti6A14V from a SBF x 5 solution: Influence of magnesium. Biomaterials 2002, 23, 2211–2220. [Google Scholar] [CrossRef]
- Barrere, F.; van Blitterswijk, C.A.; de Groot, K.; Layrolle, P. Influence of ionic strength and carbonate on the Ca-P coating formation from SBFx5 solution. Biomaterials 2002, 23, 1921–1930. [Google Scholar] [CrossRef]
- Barrere, F.; Layrolle, P.; van Blitterswijk, C.A.; de Groot, K. Biomimetic coatings on titanium: A crystal growth study of octacalcium phosphate. J. Mater. Sci. Mater. Med. 2001, 12, 529–534. [Google Scholar] [CrossRef]
- Liu, Y.; de Groot, K.; Hunziker, E.B. BMP-2 liberated from biomimetic implant coatings induces and sustains direct ossification in an ectopic rat model. Bone 2005, 36, 745–757. [Google Scholar] [CrossRef]
- Wernike, E.; Hofstetter, W.; Liu, Y.; Wu, G.; Sebald, H.J.; Wismeijer, D.; Hunziker, E.B.; Siebenrock, K.A.; Klenke, F.M. Long-term cell-mediated protein release from calcium phosphate ceramics. J. Biomed. Mater. Res. A 2009, 92, 463–474. [Google Scholar]
- Liu, Y.; Enggist, L.; Kuffer, A.F.; Buser, D.; Hunziker, E.B. The influence of BMP-2 and its mode of delivery on the osteoconductivity of implant surfaces during the early phase of osseointegration. Biomaterials 2007, 28, 2677–2686. [Google Scholar] [CrossRef]
- Wu, G.; Liu, Y.; Iizuka, T.; Hunziker, E.B. The effect of a slow mode of BMP-2 delivery on the inflammatory response provoked by bone-defect-filling polymeric scaffolds. Biomaterials 2010, 31, 7485–7493. [Google Scholar]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef]
- Liu, Y.; Hunziker, E.B.; Layrolle, P.; de Bruijn, J.D.; de Groot, K. Bone morphogenetic protein 2 incorporated into biomimetic coatings retains its biological activity. Tissue Eng. 2004, 10, 101–108. [Google Scholar] [CrossRef]
- Luong, L.N.; Hong, S.I.; Patel, R.J.; Outslay, M.E.; Kohn, D.H. Spatial control of protein within biomimetically nucleated mineral. Biomaterials 2006, 27, 1175–1186. [Google Scholar] [CrossRef]
- Yu, X.; Qu, H.; Knecht, D.A.; Wei, M. Incorporation of bovine serum albumin into biomimetic coatings on titanium with high loading efficacy and its release behavior. J. Mater. Sci. Mater. Med. 2009, 20, 287–294. [Google Scholar] [CrossRef]
- Franceschi, R.T. Biological approaches to bone regeneration by gene therapy. J. Dent. Res. 2005, 84, 1093–1103. [Google Scholar] [CrossRef]
- Bonadio, J.; Smiley, E.; Patil, P.; Goldstein, S. Localized, direct plasmid gene delivery in vivo: Prolonged therapy results in reproducible tissue regeneration. Nat. Med. 1999, 5, 753–759. [Google Scholar] [CrossRef]
- Oakes, D.A.; Lieberman, J.R. Osteoinductive applications of regional gene therapy: Ex vivo gene transfer. Clin. Orthop. Relat. Res. 2000, 379, S101–S112. [Google Scholar] [CrossRef]
- Anderson, W.F. Human gene therapy. Nature 1998, 392, 25–30. [Google Scholar] [CrossRef]
- Evans, C.H.; Robbins, P.D. Possible orthopaedic applications of gene therapy. J. Bone Jt. Surg. Am. 1995, 77, 1103–1114. [Google Scholar]
- Jenkins, D.D.; Yang, G.P.; Lorenz, H.P.; Longaker, M.T.; Sylvester, K.G. Tissue engineering and regenerative medicine. Clin. Plast. Surg. 2003, 30, 581–588. [Google Scholar] [CrossRef]
- Mahr, J.A.; Gooding, L.R. Immune evasion by adenoviruses. Immunol. Rev. 1999, 168, 121–130. [Google Scholar] [CrossRef]
- Noguchi, P. Risks and benefits of gene therapy. N. Engl. J. Med. 2003, 348, 193–194. [Google Scholar] [CrossRef]
- Wiethoff, C.M.; Middaugh, C.R. Barriers to nonviral gene delivery. J. Pharm. Sci. 2003, 92, 203–217. [Google Scholar] [CrossRef]
- Kolk, A.; Haczek, C.; Koch, C.; Vogt, S.; Kullmer, M.; Pautke, C.; Deppe, H.; Plank, C. A strategy to establish a gene-activated matrix on titanium using gene vectors protected in a polylactide coating. Biomaterials 2011, 32, 6850–6859. [Google Scholar] [CrossRef]
- Jiang, Q.H.; Liu, L.; Shen, J.W.; Peel, S.; Yang, G.L.; Zhao, S.F.; He, F.M. Influence of multilayer rhBMP-2 DNA coating on the proliferation and differentiation of MC3T3-E1 cells seeded on roughed titanium surface. J. Biomed. Mater. Res. A 2012, 100, 2766–2774. [Google Scholar]
- Chen, S.; Yang, J.; Wang, H.; Chao, Y.; Zhang, C.; Shen, J.; Zhang, P. Adenovirus encoding BMP-7 immobilized on titanium surface exhibits local delivery ability and regulates osteoblast differentiation in vitro. Arch. Oral Biol. 2013, 58, 1225–1231. [Google Scholar] [CrossRef]
- McCarty, D.M.; Monahan, P.E.; Samulski, R.J. Self-complementary recombinant adeno-associated virus (scAAV) vectors promote efficient transduction independently of DNA synthesis. Gene Ther. 2001, 8, 1248–1254. [Google Scholar]
- Dupont, K.M.; Boerckel, J.D.; Stevens, H.Y.; Diab, T.; Kolambkar, Y.M.; Takahata, M.; Schwarz, E.M.; Guldberg, R.E. Synthetic scaffold coating with adeno-associated virus encoding BMP2 to promote endogenous bone repair. Cell Tissue Res. 2012, 347, 575–588. [Google Scholar] [CrossRef]
- Ben Arav, A.; Pelled, G.; Zilberman, Y.; Kimelman-Bleich, N.; Gazit, Z.; Schwarz, E.M.; Gazit, D. Adeno-associated virus-coated allografts: A novel approach for cranioplasty. J. Tissue Eng. Regen. Med. 2012, 6, 43–50. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Wang, J.; Guo, J.; Liu, J.; Wei, L.; Wu, G. BMP-Functionalised Coatings to Promote Osteogenesis for Orthopaedic Implants. Int. J. Mol. Sci. 2014, 15, 10150-10168. https://doi.org/10.3390/ijms150610150
Wang J, Guo J, Liu J, Wei L, Wu G. BMP-Functionalised Coatings to Promote Osteogenesis for Orthopaedic Implants. International Journal of Molecular Sciences. 2014; 15(6):10150-10168. https://doi.org/10.3390/ijms150610150
Chicago/Turabian StyleWang, Jianfeng, Jing Guo, Jingsong Liu, Limin Wei, and Gang Wu. 2014. "BMP-Functionalised Coatings to Promote Osteogenesis for Orthopaedic Implants" International Journal of Molecular Sciences 15, no. 6: 10150-10168. https://doi.org/10.3390/ijms150610150
APA StyleWang, J., Guo, J., Liu, J., Wei, L., & Wu, G. (2014). BMP-Functionalised Coatings to Promote Osteogenesis for Orthopaedic Implants. International Journal of Molecular Sciences, 15(6), 10150-10168. https://doi.org/10.3390/ijms150610150




