Cytotoxic Effects of Dillapiole on Embryonic Development of Mouse Blastocysts in Vitro and in Vivo
Abstract
:1. Introduction
2. Results
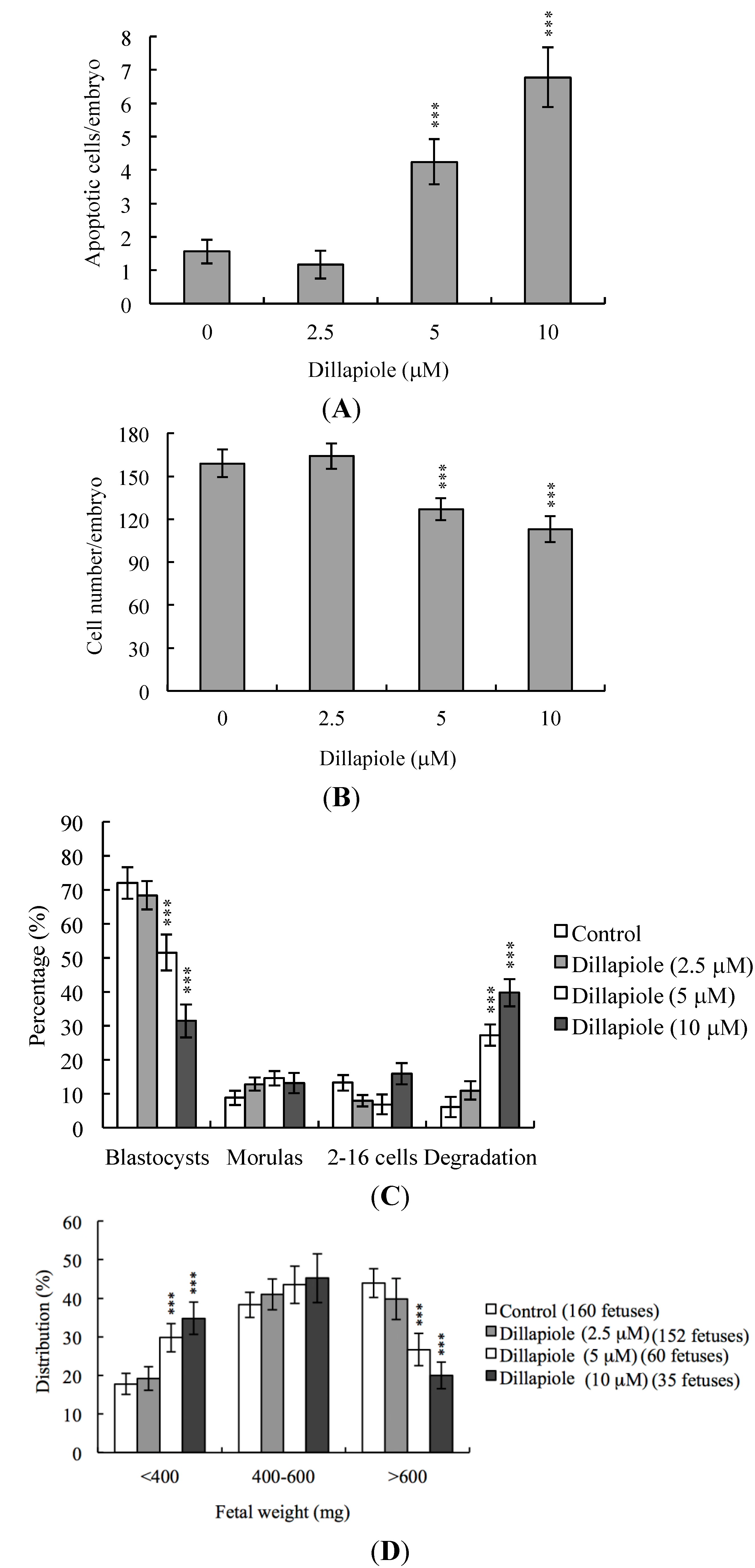
2.1. Effects of Dillapiole on Mouse Blastocysts
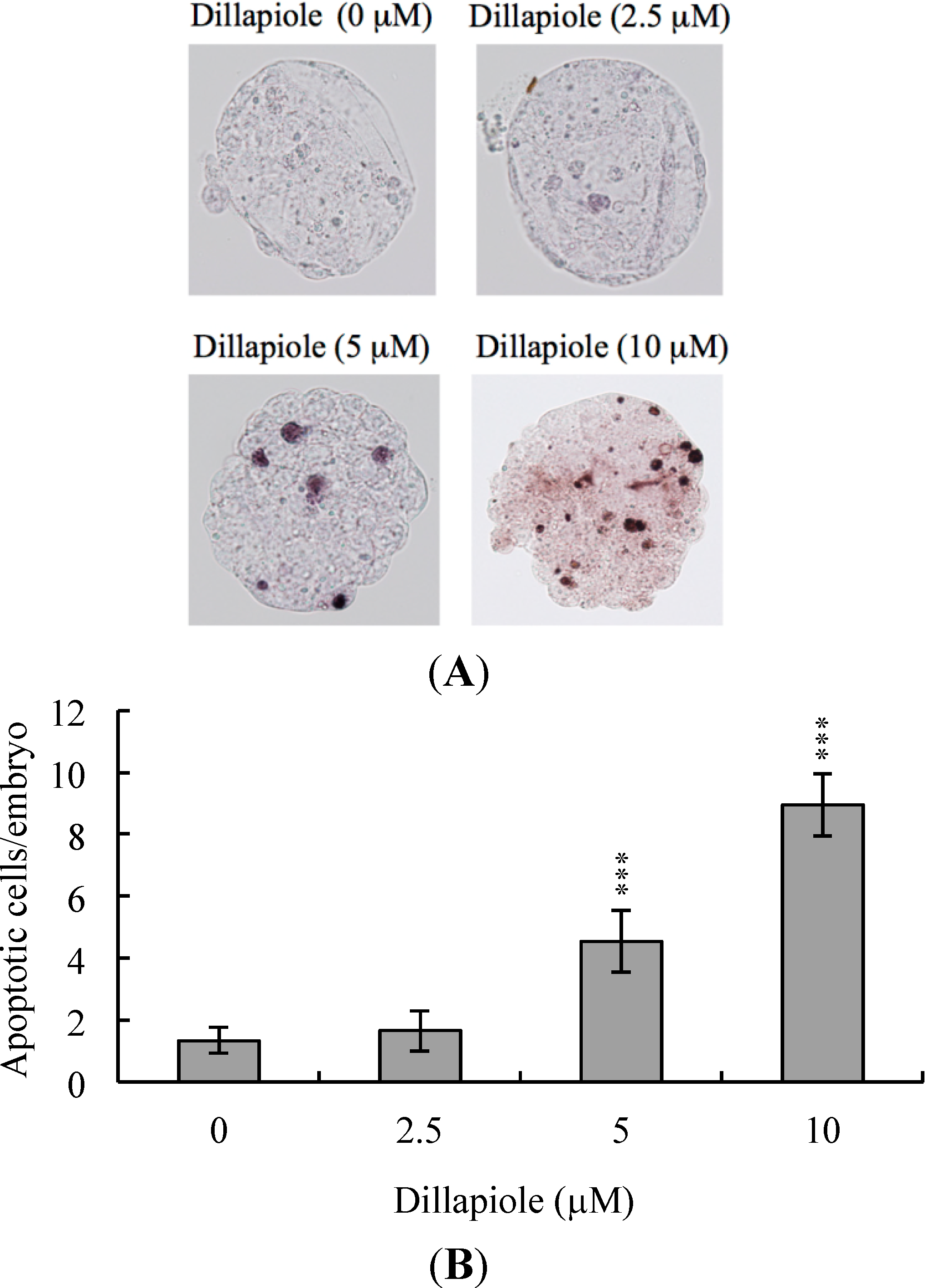
2.2. Effects of Dillapiole on Cell Proliferation
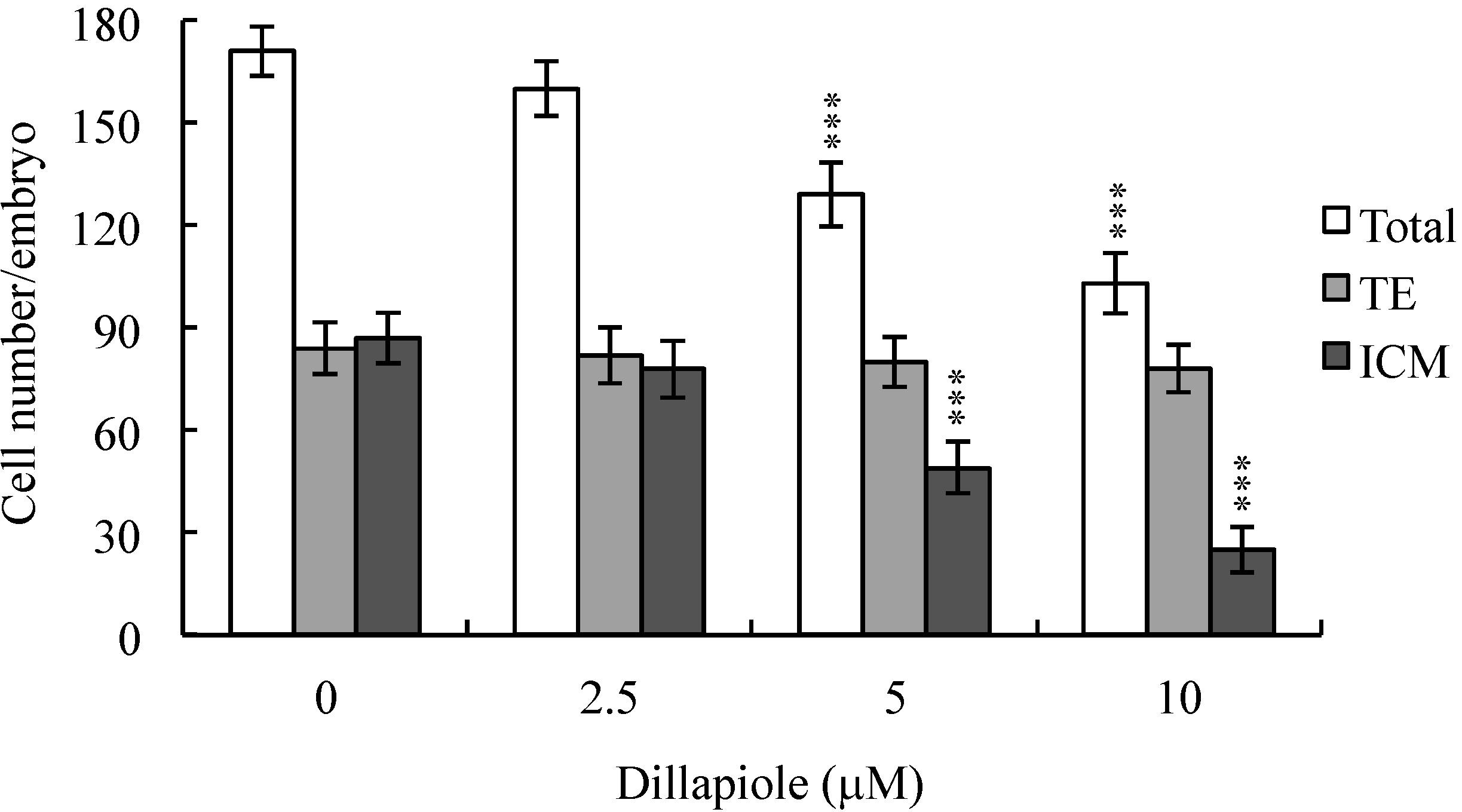
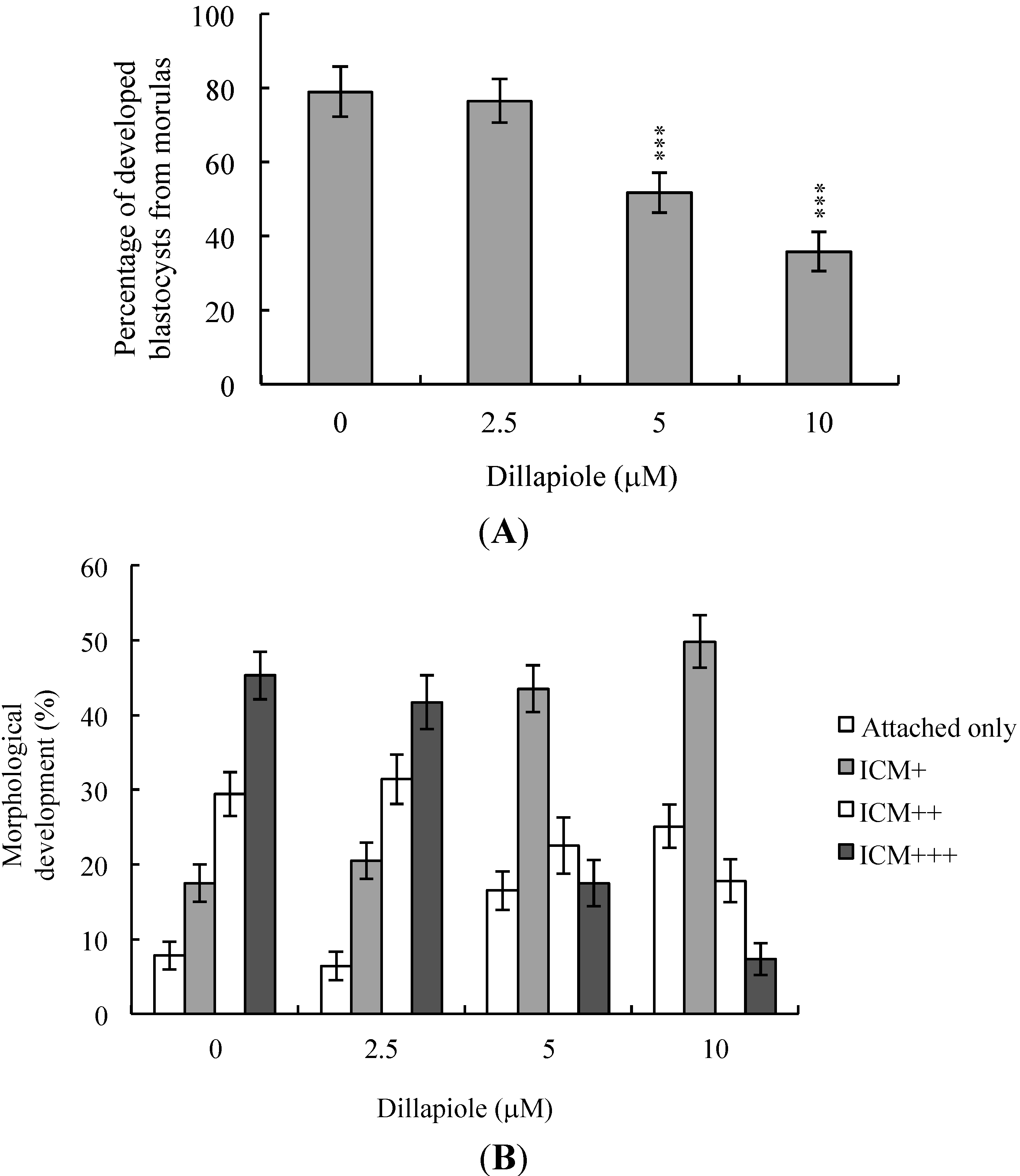
2.3. Effects of Dillapiole on Mouse Embryonic Developmental Potential in Vitro
2.4. Effects of Dillapiole on Developmental Potential of Blastocysts in Vivo
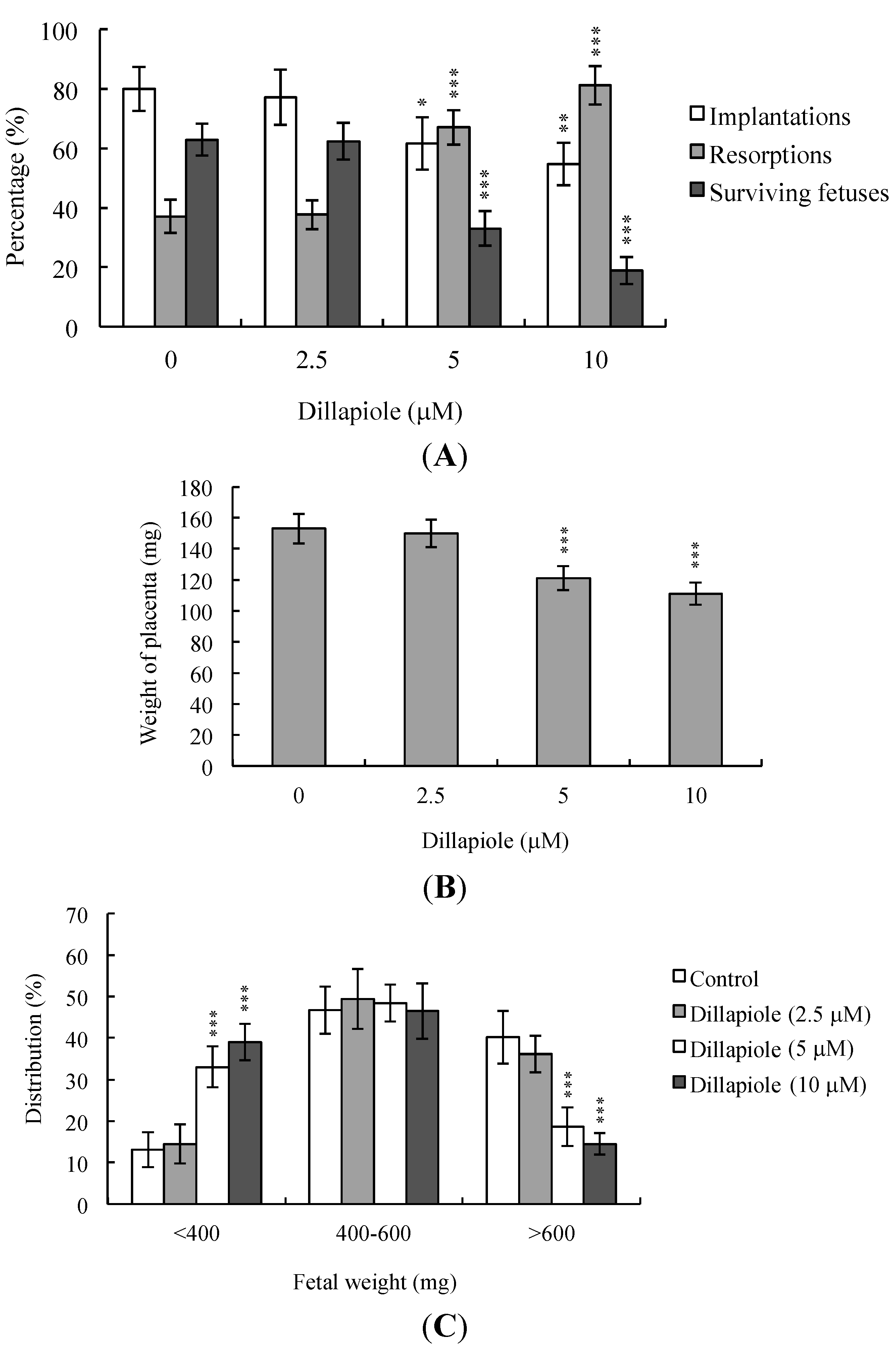
3. Discussion
4. Experimental Section
4.1. Materials
4.2. Collection of Mouse Morulas and Blastocysts
4.3. Analysis of Developed Blastocysts from Morulas
4.4. Dillapiole Treatment and TUNEL Assay
4.5. Dillapiole Treatment and Cell Proliferation
4.6. Morphological Analysis of Embryonic Development
4.7. Blastocyst Development Following Embryo Transfer
4.8. Collection of Blastocysts from Female Mice Fed Drinking Water Containing Dillapiole
4.9. Statistics
5. Conclusions
Acknowledgments
Conflicts of Interest
References
- Loc, N.H.; Bach, N.H.; Kim, T.G.; Yang, M.S. Tissue culture and expression of Escherichia coli heat-labile enterotoxin B subunit in transgenic Peperomia pellucida. Protein Expr. Purif. 2010, 72, 82–86. [Google Scholar]
- De Fatima Arrigoni-Blank, M.; Dmitrieva, E.G.; Franzotti, E.M.; Antoniolli, A.R.; Andrade, M.R.; Marchioro, M. Anti-inflammatory and analgesic activity of Peperomia pellucida (L.) HBK (Piperaceae). J. Ethnopharmacol. 2004, 91, 215–218. [Google Scholar]
- Mutee, A.F.; Salhimi, S.M.; Yam, M.F.; Lim, C.P.; Abdullah, G.Z.; Ameer, O.Z.; Abdulkarim, M.F.; Asmawi, M.Z. In vivo anti-inflammatory and in vitro antioxidant activities of Peperomia pellucida. Int. J. Pharmacol. 2010, 6, 686–690. [Google Scholar]
- Abdul Hamid, R.; Zakaria, N.; Zuraini, A. Anti-ulcer activity of aqueous etanol extract of Peperomia pellucida in Spregue-Dawley rats. Planta Med. 2007, 73, 455. [Google Scholar]
- Gao, J.; Morgan, W.A.; Sanchez-Medina, A.; Corcoran, O. The ethanol extract of Scutellaria baicalensis and the active compounds induce cell cycle arrest and apoptosis including up-regulation of p53 and Bax in human lung cancer cells. Toxicol. Appl. Pharmacol. 2011, 254, 221–228. [Google Scholar]
- De Almeida, R.R.; Souto, R.N.; Bastos, C.N.; da Silva, M.H.; Maia, J.G. Chemical variation in Piper aduncum and biological properties of its dillapiole-rich essential oil. Chem. Biodivers. 2009, 6, 1427–1434. [Google Scholar]
- Bernard, C.B.; Krishanmurty, H.G.; Chauret, D.; Durst, T.; Philogene, B.J.; Sanchez-Vindas, P.; Hasbun, C.; Poveda, L.; San Roman, L.; Arnason, J.T. Insecticidal defenses of Piperaceae from the neotropics. J. Chem. Ecol. 1995, 21, 801–814. [Google Scholar]
- Parise-Filho, R.; Pasqualoto, K.F.; Magri, F.M.; Ferreira, A.K.; da Silva, B.A.; Damiao, M.C.; Tavares, M.T.; Azevedo, R.A.; Auada, A.V.; Polli, M.C.; et al. Dillapiole as antileishmanial agent: Discovery, cytotoxic activity and preliminary SAR studies of dillapiole analogues. Arch. Pharm. 2012, 345, 934–944. [Google Scholar]
- Parise-Filho, R.; Pastrello, M.; Pereira Camerlingo, C.E.; Silva, G.J.; Agostinho, L.A.; de Souza, T.; Motter Magri, F.M.; Ribeiro, R.R.; Brandt, C.A.; Polli, M.C. The anti-inflammatory activity of dillapiole and some semisynthetic analogues. Pharm. Biol. 2011, 49, 1173–1179. [Google Scholar]
- Araujo, M.J.; Camara, C.A.; Born, F.S.; Moraes, M.M.; Badji, C.A. Acaricidal activity and repellency of essential oil from Piper aduncum and its components against Tetranychus urticae. Exp. Appl. Acarol. 2012, 57, 139–155. [Google Scholar]
- Ferreira, A.K.; de-Sa-Junior, P.L.; Pasqualoto, K.F.; de Azevedo, R.A.; Camara, D.A.; Costa, A.S.; Figueiredo, C.R.; Matsuo, A.L.; Massaoka, M.H.; Auada, A.V.; et al. Cytotoxic effects of dillapiole on MDA-MB-231 cells involve the induction of apoptosis through the mitochondrial pathway by inducing an oxidative stress while altering the cytoskeleton network. Biochimie 2014, 99, 195–207. [Google Scholar]
- Chan, W.H. Ginkgolides induce apoptosis and decrease cell numbers in mouse blastocysts. Biochem. Biophys. Res. Commun. 2005, 338, 1263–1267. [Google Scholar]
- Chan, W.H. Ginkgolide B induces apoptosis and developmental injury in mouse embryonic stem cells and blastocysts. Hum. Reprod. 2006, 21, 2985–2995. [Google Scholar]
- Chan, W.H.; Lu, H.Y.; Shiao, N.H. Effect of genistein on mouse blastocyst development in vitro. Acta Pharmacol. Sin. 2007, 28, 238–245. [Google Scholar]
- Hardy, K. Cell death in the mammalian blastocyst. Mol. Hum. Reprod. 1997, 3, 919–925. [Google Scholar]
- Hardy, K.; Stark, J.; Winston, R.M. Maintenance of the inner cell mass in human blastocysts from fragmented embryos. Biol. Reprod. 2003, 68, 1165–1169. [Google Scholar]
- Byrne, A.T.; Southgate, J.; Brison, D.R.; Leese, H.J. Analysis of apoptosis in the preimplantation bovine embryo using TUNEL. J. Reprod. Fertil. 1999, 117, 97–105. [Google Scholar]
- Hsuuw, Y.D.; Chang, C.K.; Chan, W.H.; Yu, J.S. Curcumin prevents methylglyoxal-induced oxidative stress and apoptosis in mouse embryonic stem cells and blastocysts. J. Cell. Physiol. 2005, 205, 379–386. [Google Scholar]
- Chan, W.H. Impact of genistein on maturation of mouse oocytes, fertilization, and fetal development. Reprod. Toxicol. 2009, 28, 52–58. [Google Scholar]
- Chan, W.H. Effects of citrinin on maturation of mouse oocytes, fertilization, and fetal development in vitro and in vivo. Toxicol. Lett. 2008, 180, 28–32. [Google Scholar]
- Thompson, C.B. Apoptosis in the pathogenesis and treatment of disease. Science 1995, 267, 1456–1462. [Google Scholar]
- Brill, A.; Torchinsky, A.; Carp, H.; Toder, V. The role of apoptosis in normal and abnormal embryonic development. J. Assist. Reprod. Genet. 1999, 16, 512–519. [Google Scholar]
- Lotz, K.; Proff, P.; Bienengraeber, V.; Fanghaenel, J.; Gedrange, T.; Weingaertner, J. Apoptosis as a creative agent of embryonic development of bucca, mentum and nasolacrimal duct An in vivo study in rats. J. Craniomaxillofac. Surg. 2006, 34, 8–13. [Google Scholar]
- Weingaertner, J.; Proff, P.; Bienengraeber, V.; Gedrange, T.; Fanghaenel, J.; Lotz, K. In vivo study of apoptosis as a creative agent of embryonic development of the primary nasal duct in rats. J. Craniomaxillofac. Surg. 2006, 34, 3–7. [Google Scholar]
- Huang, F.J.; Shen, C.C.; Chang, S.Y.; Wu, T.C.; Hsuuw, Y.D. Retinoic acid decreases the viability of mouse blastocysts in vitro. Hum. Reprod. 2003, 18, 130–136. [Google Scholar]
- Shang, E.H.; Wu, R.S. Aquatic hypoxia is a teratogen and affects fish embryonic development. Environ. Sci. Technol. 2004, 38, 4763–4767. [Google Scholar]
- Detmar, J.; Rabaglino, T.; Taniuchi, Y.; Oh, J.; Acton, B.M.; Benito, A.; Nunez, G.; Jurisicova, A. Embryonic loss due to exposure to polycyclic aromatic hydrocarbons is mediated by Bax. Apoptosis 2006, 11, 1413–1425. [Google Scholar]
- Huang, F.J.; Hsuuw, Y.D.; Lan, K.C.; Kang, H.Y.; Chang, S.Y.; Hsu, Y.C.; Huang, K.E. Adverse effects of retinoic acid on embryo development and the selective expression of retinoic acid receptors in mouse blastocysts. Hum. Reprod. 2006, 21, 202–209. [Google Scholar]
- Chan, W.H.; Shiao, N.H. Cytotoxic effect of CdSe quantum dots on mouse embryonic development. Acta Pharmacol. Sin. 2008, 29, 259–266. [Google Scholar]
- Chan, W.H.; Shiao, N.H. Effect of citrinin on mouse embryonic development in vitro and in vivo. Reprod. Toxicol. 2007, 24, 120–125. [Google Scholar]
- Cross, J.C.; Werb, Z.; Fisher, S.J. Implantation and the placenta: Key pieces of the development puzzle. Science 1994, 266, 1508–1518. [Google Scholar]
- Pampfer, S.; de Hertogh, R.; Vanderheyden, I.; Michiels, B.; Vercheval, M. Decreased inner cell mass proportion in blastocysts from diabetic rats. Diabetes 1990, 39, 471–476. [Google Scholar]
- Kelly, S.M.; Robaire, B.; Hales, B.F. Paternal cyclophosphamide treatment causes postimplantation loss via inner cell mass-specific cell death. Teratology 1992, 45, 313–318. [Google Scholar]
- Tam, P.P. Postimplantation development of mitomycin C-treated mouse blastocysts. Teratology 1988, 37, 205–212. [Google Scholar]
- Rojas-Martinez, R.; Arrieta, J.; Cruz-Antonio, L.; Arrieta-Baez, D.; Velazquez-Mendez, A.M.; Sanchez-Mendoza, M.E. Dillapiole, isolated from Peperomia pellucida, shows gastroprotector activity against ethanol-induced gastric lesions in Wistar rats. Molecules 2013, 18, 11327–11337. [Google Scholar]
- Hardy, K.; Handyside, A.H.; Winston, R.M. The human blastocyst: Cell number, death and allocation during late preimplantation development in vitro. Development 1989, 107, 597–604. [Google Scholar]
- Gardner, R.L.; Davies, T.J. Lack of coupling between onset of giant transformation and genome endoreduplication in the mural trophectoderm of the mouse blastocyst. J. Exp. Zool. 1993, 265, 54–60. [Google Scholar]
- Huang, F.J.; Wu, T.C.; Tsai, M.Y. Effect of retinoic acid on implantation and post-implantation development of mouse embryos in vitro. Hum. Reprod. 2001, 16, 2171–2176. [Google Scholar]
- Witschi, E. Characterization of developmental stages. Part II. Rat. In Biology Data Book, 2nd ed.; Federation of American Societies of Experimental Biologies: Washington, DC, USA, 1972; pp. 178–180. [Google Scholar]
- Armant, D.R.; Kaplan, H.A.; Lennarz, W.J. Fibronectin and laminin promote in vitro attachment and outgrowth of mouse blastocysts. Dev. Biol. 1986, 116, 519–523. [Google Scholar]
- Pampfer, S.; Wuu, Y.D.; Vanderheyden, I.; de Hertogh, R. In vitro study of the carry-over effect associated with early diabetic embryopathy in the rat. Diabetologia 1994, 37, 855–862. [Google Scholar]
- Huang, L.H.; Shiao, N.H.; Hsuuw, Y.D.; Chan, W.H. Protective effects of resveratrol on ethanol-induced apoptosis in embryonic stem cells and disruption of embryonic development in mouse blastocysts. Toxicology 2007, 242, 109–122. [Google Scholar]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Chan, W.-H. Cytotoxic Effects of Dillapiole on Embryonic Development of Mouse Blastocysts in Vitro and in Vivo. Int. J. Mol. Sci. 2014, 15, 10751-10765. https://doi.org/10.3390/ijms150610751
Chan W-H. Cytotoxic Effects of Dillapiole on Embryonic Development of Mouse Blastocysts in Vitro and in Vivo. International Journal of Molecular Sciences. 2014; 15(6):10751-10765. https://doi.org/10.3390/ijms150610751
Chicago/Turabian StyleChan, Wen-Hsiung. 2014. "Cytotoxic Effects of Dillapiole on Embryonic Development of Mouse Blastocysts in Vitro and in Vivo" International Journal of Molecular Sciences 15, no. 6: 10751-10765. https://doi.org/10.3390/ijms150610751



