Restoration of Asymmetric Dimethylarginine–Nitric Oxide Balance to Prevent the Development of Hypertension
Abstract
:1. Introduction
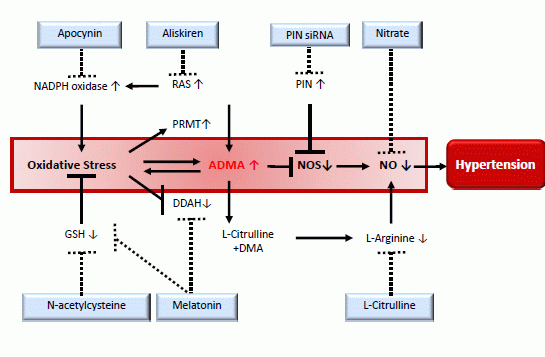
2. Asymmetric Dimethylarginine (ADMA): A Link between Nitric Oxide (NO) and Reactive Oxygen Species (ROS) in the Development of Hypertension
3. The Spontaneously Hypertensive Rat (SHR) as a Model for Developmental Programming of Hypertension
4. ADMA–NO Pathway: A Therapeutic Target for the Development of Hypertension in the SHR

5. ADMA–NO Pathway: A Therapeutic Target for Programmed Hypertension
6. Are We Ready to Apply ADMA–NO Pathway into Clinical Practice?
7. Conclusions
Acknowledgments
Author Contributions
Abbreviations
| AAR | l-arginine to ADMA ratio |
| ACE | angiotensin-converting enzyme |
| ADMA | asymmetric dimethylarginine |
| ARB | angiotensin II type 1 receptor blocker |
| CAT | cationic amino acid transporter |
| DDAH | dimethylarginine dimethylaminohydrolase |
| GSH | glutathione; |
| NAC | N-acetylcysteine |
| nNOS | neuronal nitric oxide synthase |
| NO | nitric oxide |
| NOS | nitric oxide synthase |
| PIN | protein inhibitor of neuronal nitric oxide synthase |
| PRMT | protein arginine methyltransferase |
| RAS | rennin–angiotensin system |
| ROS | reactive oxygen species |
| SDMA | symmetric dimethylarginine |
| SHR | spontaneously hypertensive rat |
| BP | blood pressure |
Conflicts of Interest
References
- Baylis, C. Nitric oxide synthase derangements and hypertension in kidney disease. Curr. Opin. Nephrol. Hypertens. 2012, 21, 1–6. [Google Scholar] [CrossRef]
- Vaziri, N.D. Roles of oxidative stress and antioxidant therapy in chronic kidney disease and hypertension. Curr. Opin. Nephrol. Hypertens. 2004, 13, 93–99. [Google Scholar] [CrossRef]
- Wilcox, C.S. Oxidative stress and nitric oxide deficiency in the kidney: A critical link to hypertension? Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005, 289, R913–R935. [Google Scholar] [CrossRef]
- Koeners, M.P.; van Faassen, E.E.; Wesseling, S.; de Sain-van der Velden, M.; Koomans, H.A.; Braam, B.; Joles, J.A. Maternal supplementation with citrulline increases renal nitric oxide in young spontaneously hypertensive rats and has long-term antihypertensive effects. Hypertension 2007, 50, 1077–1084. [Google Scholar] [CrossRef]
- Hsu, C.N.; Huang, L.T.; Lau, Y.T.; Lin, C.Y.; Tain, Y.L. The combined ratios of l-arginine, asymmetric and symmetric dimethylarginine as biomarkers in spontaneously hypertensive rats. Transl. Res. 2012, 159, 90–98. [Google Scholar] [CrossRef]
- Nuyt, A.M.; Alexander, B.T. Developmental programming and hypertension. Curr. Opin. Nephrol. Hypertens. 2009, 18, 144–152. [Google Scholar] [CrossRef]
- Racasan, S.; Braam, B.; Koomans, H.A.; Joles, J.A. Programming blood pressure in adult SHR by shifting perinatal balance of NO and reactive oxygen species toward NO: The inverted Barker phenomenon. Am. J. Physiol. Renal Physiol. 2005, 288, F626–F636. [Google Scholar]
- Tain, Y.L.; Hsieh, C.S.; Lin, I.C.; Chen, C.C.; Sheen, J.M.; Huang, L.T. Effects of maternal l-citrulline supplementation on renal function and blood pressure in offspring exposed to maternal caloric restriction: The impact of nitric oxide pathway. Nitric Oxide 2010, 23, 34–41. [Google Scholar] [CrossRef]
- Teerlink, T.; Luo, Z.; Palm, F.; Wilcox, C.S. Cellular ADMA: Regulation and action. Pharmacol. Res. 2009, 60, 448–460. [Google Scholar] [CrossRef]
- Schwedhelm, E.; Böger, R.H. The role of asymmetric and symmetric dimethylarginines in renal disease. Nat. Rev. Nephrol. 2011, 7, 275–285. [Google Scholar] [CrossRef]
- Ritz, E.; Amann, K.; Koleganova, N.; Benz, K. Prenatal programming-effects on blood pressure and renal function. Nat. Rev. Nephrol. 2011, 7, 137–144. [Google Scholar]
- Surdacki, A.; Nowicki, M.; Sandmann, J.; Tsikas, D.; Boeger, R.H.; Bode-Boeger, S.M.; Kruszelnicka-Kwiatkowska, O.; Kokot, F.; Dubiel, J.S.; Froelich, J.C. Reduced urinary excretion of nitric oxide metabolites and increased plasma levels of asymmetric dimethylarginine in men with essential hypertension. J. Cardiovasc. Pharmacol. 1999, 33, 652–658. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T. Asymmetric dimethylarginine: Clinical applications in pediatric medicine. J. Formos. Med. Assoc. 2011, 110, 70–77. [Google Scholar]
- Tain, Y.L.; Lee, W.C.; Hsu, C.N.; Lee, W.C.; Huang, L.T.; Lee, C.T.; Lin, C.Y. Asymmetric dimethylarginine is associated with developmental programming of adult kidney disease and hypertension in offspring of streptozotocin-treated mothers. PLoS One 2013, 8, e55420. [Google Scholar]
- Tain, Y.L.; Baylis, C. Determination of dimethylarginine dimethylaminohydrolase activity in the kidney. Kidney Int. 2007, 72, 886–889. [Google Scholar] [CrossRef]
- Tain, Y.L.; Freshour, G.; Dikalova, A.; Griendling, K.; Baylis, C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am. J. Physiol. Renal Physiol. 2007, 292, F1404–F1410. [Google Scholar] [CrossRef]
- Tain, Y.L.; Kao, Y.H.; Hsieh, C.S.; Chen, C.C.; Sheen, J.M.; Lin, I.C.; Huang, L.T. Melatonin blocks oxidative stress-induced increased asymmetric dimethylarginine. Free Radic. Biol. Med. 2010, 49, 1088–1098. [Google Scholar]
- Pinto, Y.M.; Paul, M.; Ganten, D. Lessons from rat models of hypertension: From Goldblatt to genetic engineering. Cardiovasc. Res. 1998, 39, 77–88. [Google Scholar] [CrossRef]
- Numabe, A.; Komatsu, A.; Frohlich, E.D. Effects of ANG converting enzyme and α1-adrenoceptor inhibition on intrarenal hemodynamics in SHR. Am. J. Physiol. 1994, 266, R1437–R1442. [Google Scholar]
- Simko, F.; Paulis, L. Melatonin as a potential antihypertensive treatment. J. Pineal Res. 2007, 42, 319–322. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Paredes, S.D.; Fuentes-Broto, L. Beneficial effects of melatonin in cardiovascular disease. Ann. Med. 2010, 42, 276–285. [Google Scholar] [CrossRef]
- Tain, Y.L.; Huang, L.T.; Lin, I.C.; Lau, Y.T.; Lin, C.Y. Melatonin prevents hypertension and increased asymmetric dimethylarginine in young spontaneous hypertensive rats. J. Pineal Res. 2010, 49, 390–398. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Huang, L.T.; Lau, Y.T. Apocynin attenuates oxidative stress and hypertension in young spontaneously hypertensive rats independent of ADMA/NO pathway. Free Radic. Res. 2012, 46, 68–76. [Google Scholar] [CrossRef]
- Wang, S.C.; Lin, K.M.; Chien, S.J.; Huang, L.T.; Hsu, C.N.; Tain, Y.L. RNA silencing targeting PIN (protein inhibitor of neuronal nitric oxide synthase) attenuates the development of hypertension in young spontaneously hypertensive rats. J. Am. Soc. Hypertens. 2014, 8, 5–13. [Google Scholar]
- Jaffrey, S.R.; Snyder, S.H. PIN: An associated protein inhibitor of neuronal nitric oxide synthase. Science 1996, 274, 774–777. [Google Scholar] [CrossRef]
- Hemmens, B.; Woschitz, S.; Pitters, E.; Klösch, B.; Völker, C.; Schmidt, K.; Mayer, B. The protein inhibitor of neuronal nitric oxide synthase (PIN): Characterization of its action on pure nitric oxide synthases. FEBS Lett. 1998, 430, 397–400. [Google Scholar] [CrossRef]
- Lee, S.K.; Arunkumar, S.; Sirajudeen, K.N.; Singh, H.J. Glutathione system in young spontaneously hypertensive rats. J. Physiol. Biochem. 2010, 66, 321–327. [Google Scholar] [CrossRef]
- Fan, N.C.; Tsai, C.M.; Hsu, C.N.; Huang, L.T.; Tain, Y.L. N-Acetylcysteine prevents hypertension via regulation of the ADMA–DDAH pathway in young spontaneously hypertensive rats. Biomed. Res. Int. 2013, 2013, 696317. [Google Scholar]
- Zhan, C.D.; Sindhu, R.K.; Vaziri, N.D. Up-regulation of kidney NAD(P)H oxidase and calcineurin in SHR: Reversal by lifelong antioxidant supplementation. Kidney Int. 2004, 65, 219–227. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Zhan, C.D.; Quiroz, Y.; Sindhu, R.K.; Vaziri, N.D. Antioxidant-rich diet relieves hypertension and reduces renal immune infiltration in spontaneously hypertensive rats. Hypertension 2003, 41, 341–346. [Google Scholar] [CrossRef]
- Gokce, N. l-Arginine and hypertension. J. Nutr. 2004, 134, 2807S–2811S. [Google Scholar]
- Romero, M.J.; Platt, D.H.; Caldwell, R.B.; Caldwell, R.W. Therapeutic use of citrulline in cardiovascular disease. Cardiovasc. Drug Rev. 2006, 24, 275–290. [Google Scholar] [CrossRef]
- Chien, S.J.; Lin, K.M.; Kuo, H.C.; Huang, C.F.; Lin, Y.J.; Huang, L.T.; Tain, Y.L. Two different approaches to restore renal nitric oxide and prevent hypertension in young spontaneously hypertensive rats: l-Citrulline and nitrate. Transl. Res. 2014, 163, 43–52. [Google Scholar] [CrossRef]
- Carlström, M.; Persson, A.E.; Larsson, E.; Hezel, M.; Scheffer, P.G.; Teerlink, T.; Weitzberg, E.; Lundberg, J.O. Dietary nitrate attenuates oxidative stress, prevents cardiac and renal injuries, and reduces blood pressure in salt-induced hypertension. Cardiovasc. Res. 2011, 89, 574–585. [Google Scholar] [CrossRef]
- Luyckx, V.A.; Bertram, J.F.; Brenner, B.M.; Fall, C.; Hoy, W.E.; Ozanne, S.E.; Vikse, B.E. Effect of fetal and child health on kidney development and long-term risk of hypertension and kidney disease. Lancet 2013, 382, 273–283. [Google Scholar] [Green Version]
- Tain, Y.L.; Sheen, J.M.; Chen, C.C.; Yu, H.R.; Tiao, M.M.; Kuo, H.C.; Huang, L.T. Maternal citrulline supplementation prevents prenatal dexamethasone-induced programmed hypertension. Free Radic. Res. 2014, 48, 580–586. [Google Scholar]
- Sherman, R.C.; Langley-Evans, S.C. Early administration of angiotensin-converting enzyme inhibitor captopril, prevents the development of hypertension programmed by intrauterine exposure to a maternal low-protein diet in the rat. Clin. Sci. 1998, 94, 373–381. [Google Scholar]
- Sherman, R.C.; Langley-Evans, S.C. Antihypertensive treatment in early postnatal life modulates prenatal dietary influences upon blood pressure in the rat. Clin. Sci. 2000, 98, 269–275. [Google Scholar] [CrossRef]
- Tain, Y.L.; Hsu, C.N.; Lin, C.Y.; Huang, L.T.; Lau, Y.T. Aliskiren prevents hypertension and reduces asymmetric dimethylarginine in young spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 670, 561–565. [Google Scholar]
- Hsu, C.N.; Lee, C.T.; Huang, L.T.; Tain, Y.L. Aliskiren in early postnatal life prevents hypertension and reduces asymmetric dimethylarginine in offspring exposed to maternal caloric restriction. J. Renin Angiotensin. Aldosterone Syst. 2014, in press. [Google Scholar]
- Böger, R.H.; Maas, R.; Schulze, F.; Schwedhelm, E. Asymmetric dimethylarginine (ADMA) as a prospective marker of cardiovascular disease and mortality—An update on patient populations with a wide range of cardiovascular risk. Pharmacol. Res. 2009, 60, 481–487. [Google Scholar]
- Taner, A.; Unlu, A.; Kayrak, M.; Tekinalp, M.; Ayhan, S.S.; Arıbaş, A.; Erdem, S.S. The value of serum asymmetric dimethylarginine levels for the determination of masked hypertension in patients with diabetes mellitus. Atherosclerosis 2013, 228, 432–437. [Google Scholar] [CrossRef]
- Jabecka, A.; Ast, J.; Bogdaski, P.; Drozdowski, M.; Pawlak-Lemaska, K.; Cielewicz, A.R.; Pupek-Musialik, D. Oral l-arginine supplementation in patients with mild arterial hypertension and its effect on plasma level of asymmetric dimethylarginine, l-citruline, l-arginine and antioxidant status. Eur. Rev. Med. Pharmacol. Sci. 2012, 16, 1665–1674. [Google Scholar]
- Kuo, H.C.; Hsu, C.N.; Huang, C.F.; Lo, M.H.; Chien, S.J.; Tain, Y.L. Urinary arginine methylation index associated with ambulatory blood pressure abnormalities in children with chronic kidney disease. J. Am. Soc. Hypertens. 2012, 6, 385–392. [Google Scholar]
- Protopsaltis, I.; Foussas, S.; Angelidi, A.; Gritzapis, A.; Sergentanis, T.N.; Matsagos, S.; Tzirogiannis, K.; Panoutsopoulos, G.I.; Dimitriadis, G.; Raptis, S.; et al. Impact of ADMA, endothelial progenitor cells and traditional cardiovascular risk factors on pulse wave velocity among prediabetic individuals. Cardiovasc. Diabetol. 2012, 11, 141. [Google Scholar] [CrossRef]
- Päivä, H.; Kähönen, M.; Lehtimäki, T.; Raitakari, O.T.; Jula, A.; Viikari, J.; Alfthan, G.; Juonala, M.; Laaksonen, R.; Hutri-Kähönen, N. Asymmetric dimethylarginine (ADMA) has a role in regulating systemic vascular tone in young healthy subjects: The cardiovascular risk in young Finns study. Am. J. Hypertens. 2008, 21, 873–878. [Google Scholar]
- Pimenta, E.; Oparil, S. Prehypertension: Epidemiology, consequences and treatment. Nat. Rev. Nephrol. 2010, 6, 21–30. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Tain, Y.-L.; Huang, L.-T. Restoration of Asymmetric Dimethylarginine–Nitric Oxide Balance to Prevent the Development of Hypertension. Int. J. Mol. Sci. 2014, 15, 11773-11782. https://doi.org/10.3390/ijms150711773
Tain Y-L, Huang L-T. Restoration of Asymmetric Dimethylarginine–Nitric Oxide Balance to Prevent the Development of Hypertension. International Journal of Molecular Sciences. 2014; 15(7):11773-11782. https://doi.org/10.3390/ijms150711773
Chicago/Turabian StyleTain, You-Lin, and Li-Tung Huang. 2014. "Restoration of Asymmetric Dimethylarginine–Nitric Oxide Balance to Prevent the Development of Hypertension" International Journal of Molecular Sciences 15, no. 7: 11773-11782. https://doi.org/10.3390/ijms150711773