Curcumin Induced Human Gastric Cancer BGC-823 Cells Apoptosis by ROS-Mediated ASK1-MKK4-JNK Stress Signaling Pathway
Abstract
:1. Introduction

2. Results and Discussion
2.1. Curcumin Inhibited Cell Proliferation in BGC-823 Cells

2.2. Curcumin Induced Oxidative Stress in BGC-823 Cells

2.3. Curcumin-Triggered Apoptosis in BGC-823 Cells Was Related with the ROS Production in BGC-823 Cells
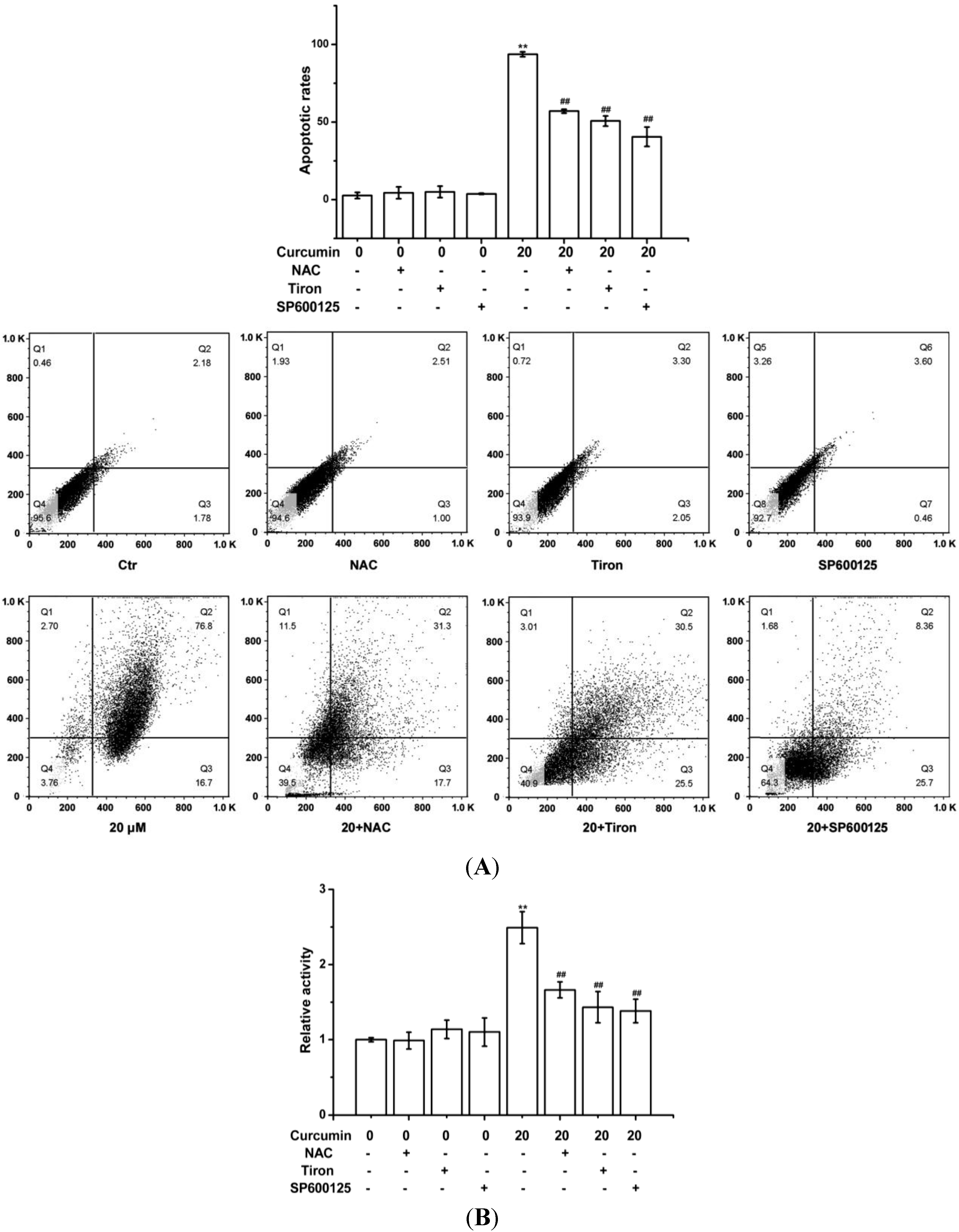
2.4. Curcumin Activated ROS-Mediated JNK Cascade in BGC-823 Cells

2.5. Discussion
3. Experimental Section
3.1. Materials
3.2. Effect of Curcumin on Cell Viability
3.3. Detection of ROS Level
3.4. Measurement of GSH/GSSG Ratio
3.5. Apoptotic Effect of Curcumin Is Assayed by Flow Cytometry
3.6. Effect of Curcumin on Caspase-3 Activity
3.7. Western Blot Analysis
3.8. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- Xiao, X.Y.; Hao, M.; Yang, X.Y.; Ba, Q.; Li, M.; Ni, S.J.; Wang, L.S.; Du, X. Licochalcone A inhibits growth of gastric cancer cells by arresting cell cycle progression and inducing apoptosis. Cancer Lett. 2011, 302, 69–75. [Google Scholar] [CrossRef]
- Shah, M.A.; Kelsen, D.P. Gastric cancer: A primer on the epidemiology and biology of the disease and an overview of the medical management of advanced disease. J. Natl. Compr. Cancer Netw. 2010, 8, 437–447. [Google Scholar]
- Logsdon, C.D.; Abbruzzese, J.L. Chemoprevention of pancreatic cancer: Ready for the clinic? Cancer Prev. Res. 2010, 3, 1375–1378. [Google Scholar]
- Dennis, T.; Fanous, M.; Mousa, S. Natural products for chemopreventive and adjunctive therapy in oncologic disease. Nutr. Cancer 2009, 61, 587–597. [Google Scholar] [CrossRef]
- Kewitz, S.; Volkmer, I.; Staege, M.S. Curcuma contra cancer? Curcumin and hodgkin’s lymphoma. Cancer Growth Metastasis 2013, 6, 35–52. [Google Scholar]
- Seeta Rama Raju, G.; Pavitra, E.; Nagaraju, G.P.; Ramesh, K.; el-Rayes, B.F.; Yu, J.S. Imaging and curcumin delivery in pancreatic cancer cell lines using PEGylated α-Gd2(MoO4)3 mesoporous particles. Dalton Trans. 2014, 43, 3330–3338. [Google Scholar]
- Sun, X.D.; Liu, X.E.; Huang, D.S. Curcumin reverses the epithelial-mesenchymal transition of pancreatic cancer cells by inhibiting the Hedgehog signaling pathway. Oncol. Rep. 2013, 29, 2401–2407. [Google Scholar]
- Glienke, W.; Maute, L.; Wicht, J.; Bergmann, L. Wilms’ tumour gene 1 (WT1) as a target in curcumin treatment of pancreatic cancer cells. Eur. J. Cancer 2009, 45, 874–880. [Google Scholar] [CrossRef]
- Huang, A.C.; Chang, C.L.; Yu, C.S.; Chen, P.Y.; Yang, J.S.; Ji, B.C.; Lin, T.P.; Chiu, C.F.; Yeh, S.P.; Huang, Y.P.; et al. Induction of apoptosis by curcumin in murine myelomonocytic leukemia WEHI-3 cells is mediated via endoplasmic reticulum stress and mitochondria-dependent pathways. Environ. Toxicol. 2013, 28, 255–266. [Google Scholar]
- Gopal, P.K.; Paul, M.; Paul, S. Curcumin induces caspase mediated apoptosis in JURKAT cells by disrupting the redox balance. Asian Pac. J. Cancer Prev. 2014, 15, 93–100. [Google Scholar] [CrossRef]
- Lin, S.S.; Huang, H.P.; Yang, J.S.; Wu, J.Y.; Hsia, T.C.; Lin, C.C.; Lin, C.W.; Kuo, C.L.; Gibson Wood, W.; Chung, J.G. DNA damage and endoplasmic reticulum stress mediated curcumin-induced cell cycle arrest and apoptosis in human lung carcinoma A-549 cells through the activation caspases cascade- and mitochondrial-dependent pathway. Cancer Lett. 2008, 272, 77–90. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, B.; Gan, L.; Wang, Z.H.; Yu, B.C.; Liu, L.L.; Zheng, Q.S.; Wang, Z.P. Involvement of the mitochondrion-dependent and the endoplasmic reticulum stress-signaling pathways in isoliquiritigenin-induced apoptosis of HeLa cell. Biomed. Environ. Sci. 2013, 26, 268–276. [Google Scholar]
- Assefa, Z.; Vantieghem, A.; Garmyn, M.; Declercq, W.; Vandenabeele, P.; Vandenheede, J.R.; Bouillon, R.; Merlevede, W.; Agostinis, P. p38 mitogen-activated protein kinase regulates a novel, caspase-independent pathway for the mitochondrial cytochrome c release in ultraviolet B radiation-induced apoptosis. J. Biol. Chem. 2000, 275, 21416–21421. [Google Scholar] [CrossRef]
- Navarro, R.; Busnadiego, I.; Ruiz-Larrea, M.B.; Ruiz-Sanz, J.I. Superoxide anions are involved in doxorubicin-induced ERK activation in hepatocyte cultures. Ann. N. Y. Acad. Sci. 2006, 1090, 419–428. [Google Scholar] [CrossRef]
- Matsuzawa, A.; Ichijo, H. Stress-responsive protein kinases in redox-regulated apoptosis signaling. Antioxid. Redox Signal. 2005, 7, 472–481. [Google Scholar] [CrossRef]
- Tournier, C.; Hess, P.; Yang, D.D.; Xu, J.; Turner, T.K.; Nimnual, A.; Bar-Sagi, D.; Jones, S.N.; Flavell, R.A.; Davis, R.J. Requirement of JNK for stress-induced activation of the cytochrome c-mediated death pathway. Science 2000, 288, 870–874. [Google Scholar] [CrossRef]
- El-Najjar, N.; Chatila, M.; Moukadem, H.; Vuorela, H.; Ocker, M.; Gandesiri, M.; Schneider-Stock, R.; Gali-Muhtasib, H. Reactive oxygen species mediate thymoquinone-induced apoptosis and activate ERK and JNK signaling. Apoptosis 2010, 15, 183–195. [Google Scholar]
- Ramachandran, C.; Rodriguez, S.; Ramachandran, R.; Nair, P.K.R.; Fonseca, H.; Khatib, Z.; Escalon, E.; Melnick, S.J. Expression profiles of apoptotic genes induced by curcumin in human breast cancer and mammary epithelial cell lines. Anticancer Res. 2005, 25, 3293–3302. [Google Scholar]
- Woo, J.H.; Kim, Y.H.; Choi, Y.J.; Kim, D.G.; Lee, K.S.; Bae, J.H.; Min, D.S.; Chang, J.S.; Jeong, Y.J.; Lee, Y.H.; et al. Molecular mechanisms of curcumin-induced cytotoxicity: Induction of apoptosis through generation of reactive oxygen species, down-regulation of Bcl-XL and IAP, the release of cytochrome c and inhibition of Akt. Carcinogenesis 2003, 24, 1199–1208. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Zhu, S.; Wen, J.; Farris, A.B.; Adsay, V.N.; Diaz, R.; Snyder, J.P.; Mamoru, S.; El-Rayes, B.F. Novel synthetic curcumin analogues EF31 and UBS109 are potent DNA hypomethylating agents in pancreatic cancer. Cancer Lett. 2013, 341, 195–203. [Google Scholar] [CrossRef]
- Azuine, M.A.; Kayal, J.J.; Bhide, S.V. Protective role of aqueous turmeric extract against mutagenicity of direct-acting carcinogens as well as benzo [alpha] pyrene-induced genotoxicity and carcinogenicity. J. Cancer Res. Clin. Oncol. 1992, 118, 447–452. [Google Scholar] [CrossRef]
- Subramaniam, D.; May, R.; Sureban, S.M.; Lee, K.B.; George, R.; Kuppusamy, P.; Ramanujam, R.P.; Hideg, K.; Dieckgraefe, B.K.; Houchen, C.W.; et al. Diphenyl difluoroketone: A curcumin derivative with potent in vivo anticancer activity. Cancer Res. 2008, 68, 1962–1969. [Google Scholar] [CrossRef]
- Moragoda, L.; Jaszewski, R.; Majumdar, A.P. Curcumin induced modulation of cell cycle and apoptosis in gastric and colon cancer cells. Anticancer Res. 2001, 21, 873–878. [Google Scholar]
- Qin, H.B.; Wei, L.; Zhang, J.W.; Tang, J.M. Study on functions and mechanism of curcumin in inducing gastric carcinoma BGC apoptosis. Xi Bao Yu Fen Zi Mian Yi Xue Za Zhi 2011, 27, 1227–1230. [Google Scholar]
- Cai, X.Z.; Huang, W.Y.; Qiao, Y.; Du, S.Y.; Chen, Y.; Chen, D.; Yu, S.; Che, R.C.; Liu, N.; Jiang, Y. Inhibitory effects of curcumin on gastric cancer cells: A proteomic study of molecular targets. Phytomedicine 2013, 20, 495–505. [Google Scholar] [CrossRef]
- Tu, S.P.; Jin, H.; Shi, J.D.; Zhu, L.M.; Suo, Y.; Lu, G.; Liu, A.; Wang, T.C.; Yang, C.S. Curcumin induces the differentiation of myeloid-derived suppressor cells and inhibits their interaction with cancer cells and related tumor growth. Cancer Prev. Res. 2012, 5, 205–215. [Google Scholar] [CrossRef]
- Gao, C.; Ding, Z.; Liang, B.; Chen, N.; Cheng, D. Study on the effects of curcumin on angiogenesis. Zhong Yao Cai 2003, 26, 499–502. [Google Scholar]
- Cai, X.Z.; Wang, J.; Li, X.D.; Wang, G.L.; Liu, F.N.; Cheng, M.S.; Li, F. Curcumin suppresses proliferation and invasion in human gastric cancer cells by downregulation of PAK1 activity and cyclin D1 expression. Cancer Biol. Ther. 2009, 8, 1360–1368. [Google Scholar] [CrossRef]
- Yu, L.L.; Wu, J.G.; Dai, N.; Yu, H.G.; Si, J.M. Curcumin reverses chemoresistance of human gastric cancer cells by downregulating the NF-kappaB transcription factor. Oncol. Rep. 2011, 26, 1197–1203. [Google Scholar]
- Koo, J.Y.; Kim, H.J.; Jung, K.O.; Park, K.Y. Curcumin inhibits the growth of AGS human gastric carcinoma cells in vitro and shows synergism with 5-fluorouracil. J. Med. Food 2004, 7, 117–121. [Google Scholar] [CrossRef]
- Turrens, J.F. Mitochondrial formation of reactive oxygen species. J. Physiol. 2003, 552, 335–344. [Google Scholar] [CrossRef]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef]
- Jagtap, S.; Meganathan, K.; Wagh, V.; Winkler, J.; Hescheler, J.; Sachinidis, A. Chemoprotective mechanism of the natural compounds, epigallocatechin-3-O-gallate, quercetin and curcumin against cancer and cardiovascular diseases. Curr. Med. Chem. 2009, 16, 1451–1462. [Google Scholar] [CrossRef]
- Chetram, M.A.; Bethea, D.A.; Odero-Marah, V.A.; Don-Salu-Hewage, A.S.; Jones, K.J.; Hinton, C.V. ROS-mediated activation of AKT induces apoptosis via pVHL in prostate cancer cells. Mol. Cell. Biochem. 2013, 376, 63–71. [Google Scholar] [CrossRef]
- Efferth, T.; Giaisi, M.; Merling, A.; Krammer, P.H.; Li-Weber, M. Artesunate induces ROS-mediated apoptosis in doxorubicin-resistant T leukemia cells. PLoS One 2007, 2, e693. [Google Scholar] [CrossRef]
- Johnson, N.L.; Gardner, A.M.; Diener, K.M.; Lange-Carter, C.A.; Gleavy, J.; Jarpe, M.B.; Minden, A.; Karin, M.; Zon, L.I.; Johnson, G.L. Signal transduction pathways regulated by mitogen-activated/extracellular response kinase kinase kinase induce cell death. J. Biol. Chem. 1996, 271, 3229–3237. [Google Scholar] [CrossRef]
- Li, Y.; Liu, Y.; Fu, Y.; Wei, T.; le Guyader, L.; Gao, G.; Liu, R.-S.; Chang, Y.-Z.; Chen, C. The triggering of apoptosis in macrophages by pristine graphene through the MAPK and TGF-β signaling pathways. Biomaterials 2012, 33, 402–411. [Google Scholar] [CrossRef]
- Han, X.; Xu, B.; Beevers, C.S.; Odaka, Y.; Chen, L.; Liu, L.; Luo, Y.; Zhou, H.; Chen, W.; Shen, T.; et al. Curcumin inhibits protein phosphatases 2A and 5, leading to activation of mitogen-activated protein kinases and death in tumor cells. Carcinogenesis 2012, 33, 868–875. [Google Scholar]
- Collett, G.P.; Campbell, F.C. Curcumin induces c-Jun N-terminal kinase-dependent apoptosis in HCT116 human colon cancer cells. Carcinogenesis 2004, 25, 2183–2189. [Google Scholar] [CrossRef]
- Weir, N.M.; Selvendiran, K.; Kutala, V.K.; Tong, L.; Vishwanath, S.; Rajaram, M.; Tridandapani, S.; Anant, S.; Kuppusamy, P. Curcumin induces G2/M arrest and apoptosis in cisplatin-resistant human ovarian cancer cells by modulating Akt and p38 MAPK. Cancer Biol. Ther. 2007, 6, 178–184. [Google Scholar] [CrossRef]
- Wang, W.Z.; Li, L.; Liu, M.Y.; Jin, X.B.; Mao, J.W.; Pu, Q.H.; Meng, M.J.; Chen, X.G.; Zhu, J.Y. Curcumin induces FasL-related apoptosis through p38 activation in human hepatocellular carcinoma Huh7 cells. Life Sci. 2013, 92, 352–358. [Google Scholar] [CrossRef]
- Guo, Y.; Shan, Q.; Gong, Y.; Lin, J.; Shi, F.; Shi, R.; Yang, X. Curcumin induces apoptosis via simultaneously targeting AKT/mTOR and RAF/MEK/ERK survival signaling pathways in human leukemia THP-1 cells. Pharmazie 2014, 69, 229–233. [Google Scholar]
- Hou, J.; Wang, D.; Zhang, R.; Wang, H. Experimental therapy of hepatoma with artemisinin and its derivatives: In vitro and in vivo activity, chemosensitization, and mechanisms of action. Clin. Cancer Res. 2008, 14, 5519–5530. [Google Scholar] [CrossRef]
- Yuan, X.; Zhang, B.; Chen, N.; Chen, X.Y.; Liu, L.L.; Zheng, Q.S.; Wang, Z.P. Isoliquiritigenin treatment induces apoptosis by increasing intracellular ROS levels in HeLa cells. J. Asian Nat. Prod. Res. 2012, 14, 789–798. [Google Scholar] [CrossRef]
- Yuan, X.; Yu, B.; Wang, Y.; Jiang, J.; Liu, L.; Zhao, H.; Qi, W.; Zheng, Q. Involvement of endoplasmic reticulum stress in isoliquiritigenin-induced SKOV-3 cell apoptosis. Recent Pat. Anticancer Drug Discov. 2013, 8, 191–199. [Google Scholar] [CrossRef]
- Zheng, Q.S.; Zheng, R.L. Effects of ascorbic acid and sodium selenite on growth and redifferentiation in human hepatoma cells and its mechanisms. Pharmazie 2002, 57, 265–269. [Google Scholar]
- Park, H.Y.; Kim, G.Y.; Moon, S.K.; Kim, W.J.; Yoo, Y.H.; Choi, Y.H. Fucoidan inhibits the proliferation of human urinary bladder cancer t24 cells by blocking cell cycle progression and inducing apoptosis. Molecules 2014, 19, 5981–5998. [Google Scholar] [CrossRef]
- Kim, S.D.; Moon, C.K.; Eun, S.Y.; Ryu, P.D.; Jo, S.A. Identification of ASK1, MKK4, JNK, c-Jun, and caspase-3 as a signaling cascade involved in cadmium-induced neuronal cell apoptosis. Biochem. Biophys. Res. Commun. 2005, 328, 326–334. [Google Scholar] [CrossRef]
- Nagaraju, G.P.; Nalla, A.K.; Gupta, R.; Mohanam, S.; Gujrati, M.; Dinh, D.H.; Rao, J.S. siRNA-mediated downregulation of MMP-9 and uPAR in combination with radiation induces G2/M cell-cycle arrest in Medulloblastoma. Mol. Cancer Res. 2011, 9, 51–66. [Google Scholar] [CrossRef]
© 2014 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Liang, T.; Zhang, X.; Xue, W.; Zhao, S.; Zhang, X.; Pei, J. Curcumin Induced Human Gastric Cancer BGC-823 Cells Apoptosis by ROS-Mediated ASK1-MKK4-JNK Stress Signaling Pathway. Int. J. Mol. Sci. 2014, 15, 15754-15765. https://doi.org/10.3390/ijms150915754
Liang T, Zhang X, Xue W, Zhao S, Zhang X, Pei J. Curcumin Induced Human Gastric Cancer BGC-823 Cells Apoptosis by ROS-Mediated ASK1-MKK4-JNK Stress Signaling Pathway. International Journal of Molecular Sciences. 2014; 15(9):15754-15765. https://doi.org/10.3390/ijms150915754
Chicago/Turabian StyleLiang, Tao, Xiaojian Zhang, Wenhua Xue, Songfeng Zhao, Xiang Zhang, and Jianying Pei. 2014. "Curcumin Induced Human Gastric Cancer BGC-823 Cells Apoptosis by ROS-Mediated ASK1-MKK4-JNK Stress Signaling Pathway" International Journal of Molecular Sciences 15, no. 9: 15754-15765. https://doi.org/10.3390/ijms150915754



