Synthesis and Degradation of Schiff Bases Containing Heterocyclic Pharmacophore
Abstract
:1. Introduction
2. Results and Discussion
2.1. Synthesis of Schiff Bases
2.2. Characterization of Compounds

2.2.1. Physico-Chemical Analysis of Schiff Bases
2.2.2. Spectroscopic Analysis

2.2.3. Thermal Analysis

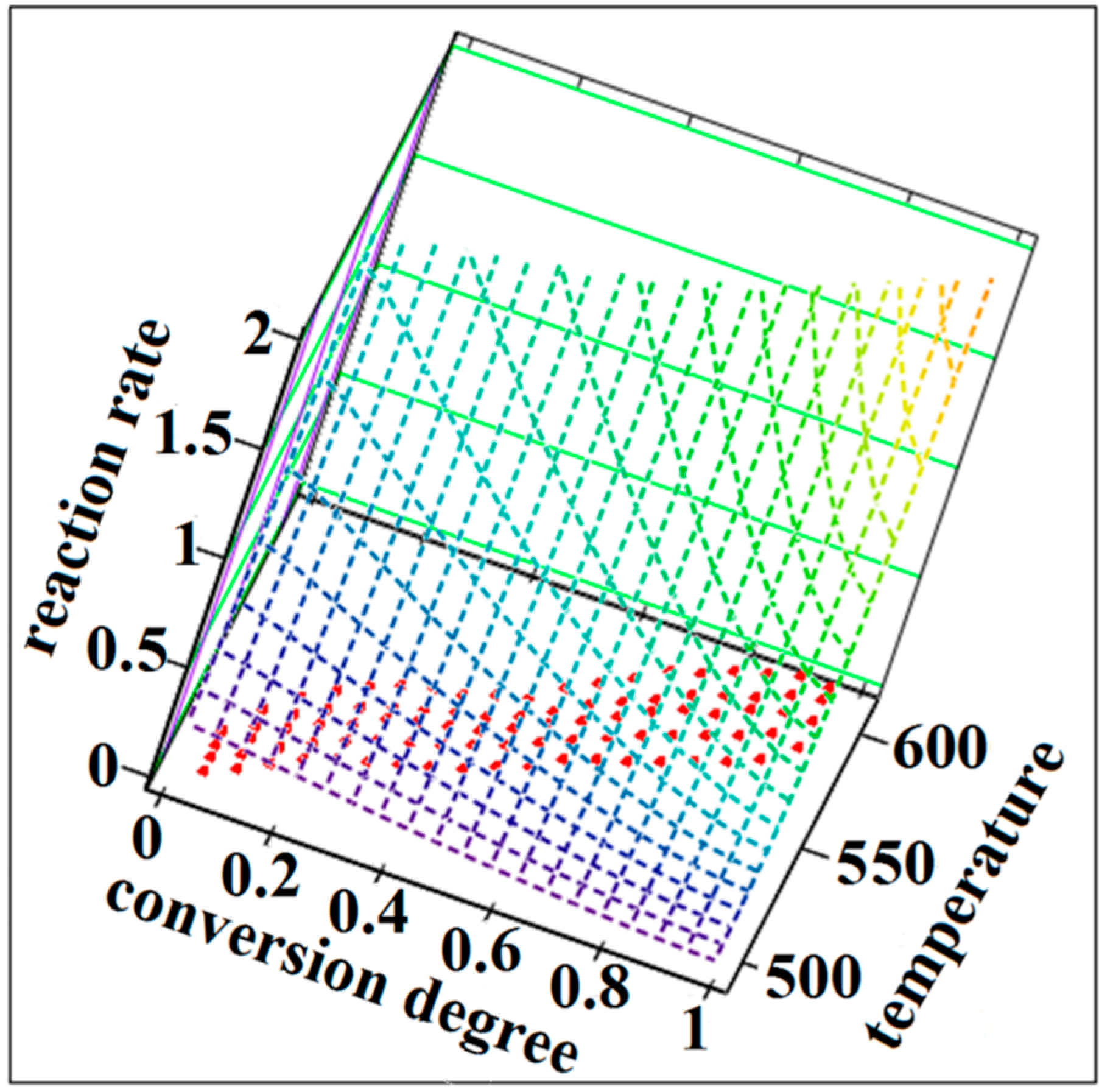
2.2.4. Kinetic Study

Flynn-Wall-Ozawa Method (FWO)


| Ea (kJ/mol) | Conversion Degree (α) | (kJ/mol) | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.05 | 0.1 | 0.15 | 0.2 | 0.25 | 0.3 | 0.35 | 0.4 | 0.45 | 0.5 | 0.55 | 0.6 | 0.65 | 0.7 | 0.75 | 0.8 | 0.85 | 0.9 | 0.95 | |||
| SB1 | FR | 116.8 | 122.9 | 149.4 | 144.4 | 135.7 | 136.6 | 137.8 | 139.4 | 142.2 | 140.4 | 136.2 | 141.5 | 141.1 | 140.1 | 140.9 | 136.8 | 131.2 | 134.4 | 138.0 | 137.2 ± 1.7 |
| KAS | 122.1 | 126.4 | 162.5 | 155.9 | 159.3 | 165.1 | 160.1 | 160.7 | 160.0 | 160.9 | 158.8 | 154.7 | 154.6 | 156.8 | 152.9 | 151.4 | 149.6 | 147.4 | 152.9 | 153.3 ± 2.6 | |
| FWO | 123.4 | 127.8 | 162.3 | 156.1 | 159.5 | 165.1 | 160.3 | 160.9 | 160.4 | 161.3 | 159.3 | 155.4 | 155.4 | 157.5 | 153.9 | 152.5 | 150.9 | 148.9 | 154.2 | 153.9 ± 2.5 | |
| SB2 | FR | 114.7 | 120.8 | 121.8 | 118.6 | 115.3 | 117.1 | 118.9 | 119.5 | 120.2 | 118.2 | 118.1 | 123.3 | 126.9 | 106.7 | 106.9 | 106.5 | 104.2 | 104.3 | 96.0 | 114.6 ± 1.9 |
| KAS | 119.8 | 118.3 | 117.3 | 116.5 | 116.8 | 118.7 | 116.3 | 116.8 | 117.6 | 117.2 | 110.1 | 114.3 | 116.2 | 97.6 | 97.9 | 95.4 | 93.7 | 95.4 | 95.2 | 110.1 ± 2.3 | |
| FWO | 121.7 | 120.5 | 119.6 | 118.9 | 119.3 | 121.1 | 118.9 | 119.5 | 120.3 | 119.9 | 113.3 | 117.4 | 119.3 | 101.7 | 102.1 | 99.73 | 98.19 | 99.91 | 99.92 | 113.2 ± 2.1 | |
Friedman Method (FR)

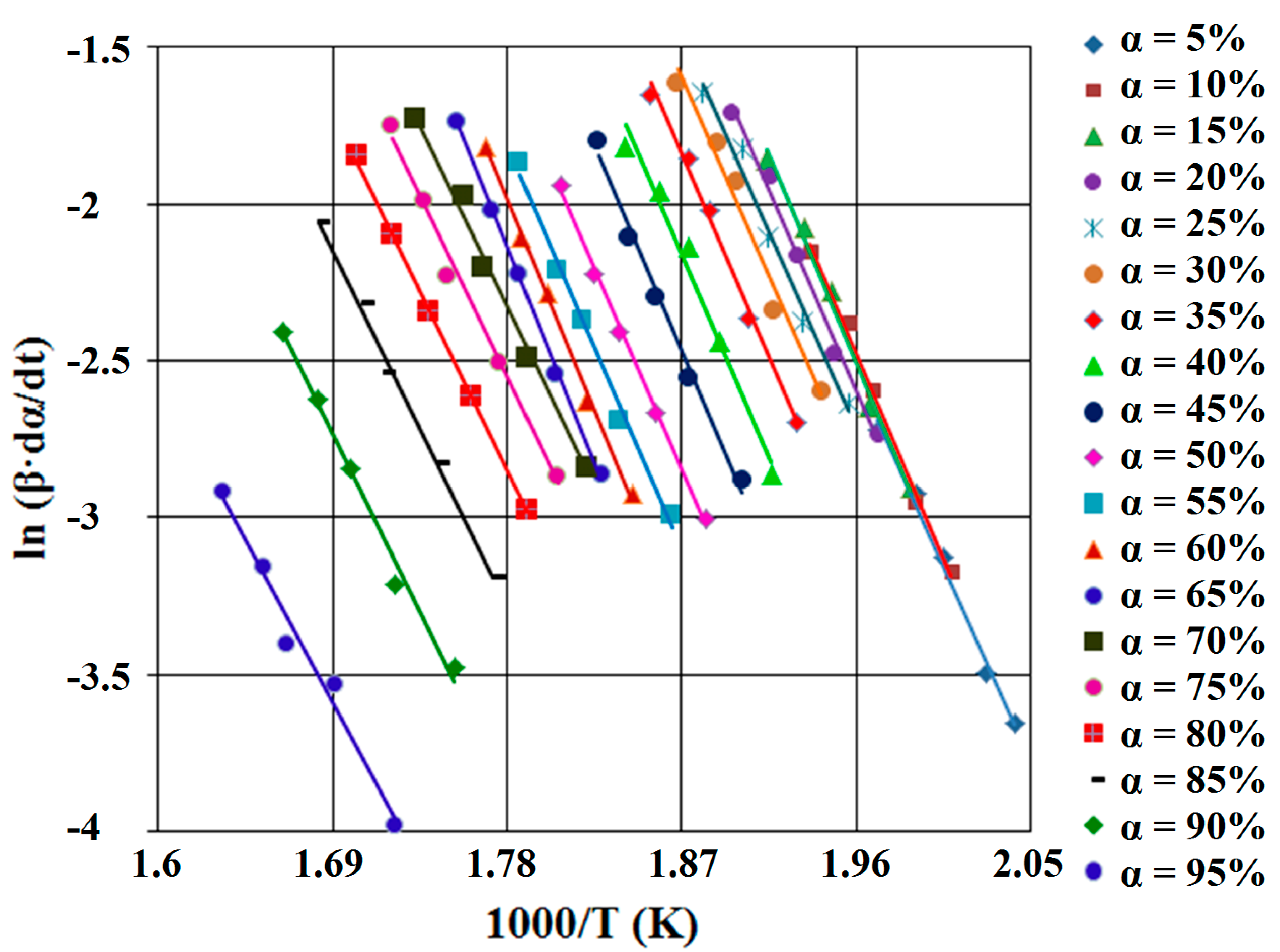
Kissinger-Akahira-Sunose Method (KAS)
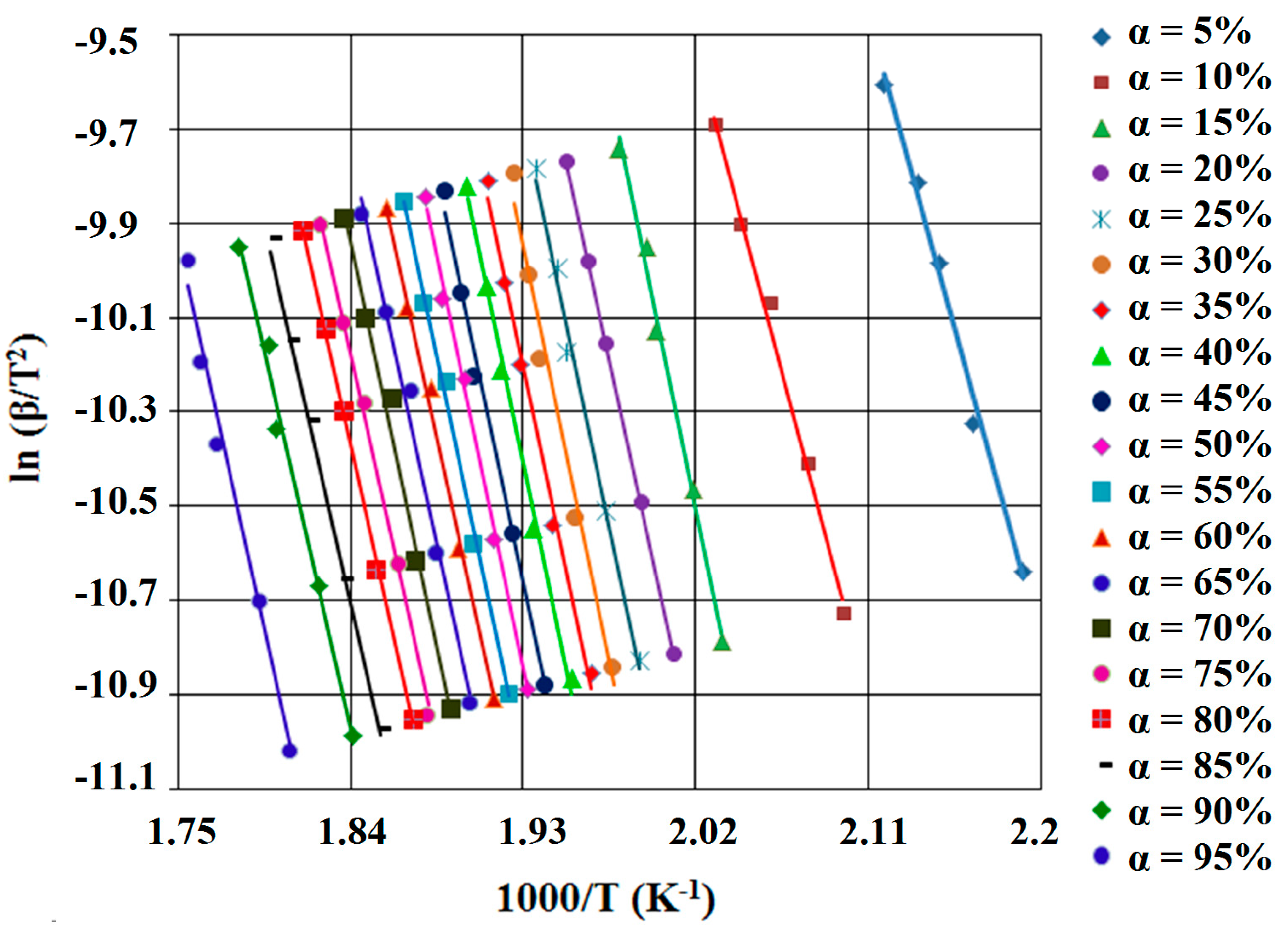

| Process | λ (%) | Ea (kJ·mol−1) | A (s−1) | n | m | Šestak-Berggren Equation | (kJ·mol−1) | |
|---|---|---|---|---|---|---|---|---|
| SB1 | 1 | 66.2 | 124.0 ± 7.7 | 1.33 × 1010 | 1/3 | - | (1 − α)1/3 | 135.1 ± 10.7 |
| 2 | 31.4 | 152.2 ± 2.7 | 3.19 × 1015 | 1 | - | (1 − α) | ||
| 3 | 2.4 | 214.5 ± 8.3 | 9.95 × 1018 | 1 | 1 | (1 − α)·α | ||
| SB2 | 1 | 89.9 | 111.4 ± 7.2 | 1.66 × 1010 | 1/2 | - | (1 − α)1/2 | 109.9 ± 8.2 |
| 2 | 9.4 | 104.2 ± 1.1 | 2.82 × 1010 | - | 1/3 | α1/3 |

3. Experimental Section
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sumangala, V.; Poojary, B.; Chidananda, N.; Arulmoli, T.; Shenoy, S. Synthesis and biological evaluation of some Schiff bases of 4-amino-5-(4-methylsulfonyl)benzyl-2,4-dihydro-3H-1,2,4-triazole-3-thione. Med. Chem. Res. 2013, 22, 2921–2928. [Google Scholar] [CrossRef]
- Aswathanarayanappa, C.; Bheemappa, E.; Bodke, Y.D.; Biradar, S.; Sindhe, A.; Peethambar, S.K.; Ningegowda, R. Synthesis, in vitro and in vivo anti-hyperglycemic activity of 1,2,4-triazolebenzylidene and 1,3,4-thiadiazole derivatives. Int. J. Pharm. Tech. Res. 2014, 6, 327–335. [Google Scholar]
- Sachdeva, H.; Dwivedi, D.; Arya, K.; Khaturia, S.; Saroj, R. Synthesis, anti-inflammatory activity, and QSAR study of some Schiff bases derived from 5-mercapto-3-(4'-pyridyl)-4H-1,2,4-triazol-4-yl-thiosemicarbazide. Med. Chem. Res. 2013, 22, 4953–4963. [Google Scholar] [CrossRef]
- Sondhi, S.M.; Arya, S.; Rani, R.; Kumar, N.; Roy, P. Synthesis, anti-inflammatory and anticancer activity evaluation of some mono- and bis-Schiff’s bases. Med. Chem. Res. 2012, 21, 3620–3628. [Google Scholar] [CrossRef]
- Sun, X.; Bai, Y.; Liu, Y.; Chen, B. Synthesis, structure and biological activities of 3-substituted phenoxymethyl-4-amino-1,2,4-triazol-5-thione Schiff bases. Acta Chim. Sin. 2010, 68, 788–792. [Google Scholar]
- Küçükgüzel, I.; Güniz Küçükgüzel, Ş.; Rollas, S.; Ötük-Saniş, G.; Özdemir, O.; Bayrak, I.; Altuǧ, T.; Stables, J.P. Synthesis of some 3-(arylalkylthio)-4-alkyl/aryl-5-(4-aminophenyl)-4H-1,2,4-triazole derivatives and their anticonvulsant activity. Farmaco 2004, 59, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Basavapatna, N.; Kumar, P.; Kikkeri, N.M.; Lingappa, M. Synthesis and antiproliferative activity of some new fluorinated Schiff bases derived from 1,2,4-triazoles. J. Fluor. Chem. 2013, 156, 15–20. [Google Scholar] [CrossRef]
- Ansari, K.R.; Quraishi, M.A.; Singh, A. Schiff’s base of pyridyl substituted triazoles as new and effective corrosion inhibitors for mild steel in hydrochloric acid solution. Corros. Sci. 2014, 79, 5–15. [Google Scholar] [CrossRef]
- Brandt, C.D.; Kitchen, K.D.; Beckmann, U.; White, N.G.; Jameson, G.B.; Brooker, S. Synthesis and structures of 3,5-disubstituted 1,2,4-triazole head units and incorporation of 3,5-dibenzoyl-1,2,4-triazolate into new [2 + 2] Schiff-base macrocyclic complexes. Supramol. Chem. 2007, 19, 17–27. [Google Scholar] [CrossRef]
- Ledeti, I.V.; Bercean, V.N.; Badea, V.; Balan, M.; Csunderlik, C. The Alkylation of 1H-5-mercapto-3-phenyl-1,2,4-triazole and 4H-4-amino-5-mercapto-3-phenyl-1,2,4-triazole. Rev. Chim. Buchar. 2010, 61, 833–837. [Google Scholar]
- Bercean, V.N.; Ledeti, I.V.; Badea, V.; Balan, M.; Csunderlik, C. New heterocyclic tioether derived from 3-substituted-4H-4-amino-5-mercapto-1,2,4-triazoles and succinic acid. Rev. Chim. Buchar. 2010, 61, 1028–1030. [Google Scholar]
- Ledeti, I.V.; Bercean, V.N.; Tanase, I.M.; Creanga, A.A.; Badea, V.; Csunderlik, C. New azomethine derivatives of 3-substituted-4H-4-amino-5-ethoxycarbonyl-methylsulfanyl-1,2,4-triazoles as potential anti-inflammatory agents. Rev. Chim. Buchar. 2010, 61, 935–937. [Google Scholar]
- Mobinikhaledi, A.; Forughifar, N.; Kahlor, M. An efficient synthesis of Schiff bases containing benzimidazole moiety catalyzed by transition metal nitrates. Turk. J. Chem. 2010, 34, 367–373. [Google Scholar]
- Foroughifar, N.; Mobinikhaledi, A.; Ebrahimi, S.; Moghanian, H.; Fard, M.A.B.; Kalhor, M. Synthesis of a new class of azathia crown macrocycles containing two 1,2,4-triazole or two 1,3,4-thiadiazole rings as subunits. Tetrahedron Lett. 2009, 50, 836–839. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Q.; Li, Q.; Ma, C. Syntheses and characterization of triorganotin(IV) complexes of Schiff base derive from 4-amino-5-phenyl-4H-1,2,4-triazole-3-thiol and 5-amino-1,3,4-thiadiazole-2-thiol with p-phthalaldehyde. Inorg. Chim. Acta 2009, 362, 2762–2769. [Google Scholar] [CrossRef]
- Bayrak, H.; Demirbas, A.; Demirbas, N.; Karaoglu, SA. Synthesis of some new 1,2,4-triazoles starting from isonicotinic acid hydrazide and evaluation of their antimicrobial activities. Eur. J. Med. Chem. 2009, 44, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Khanmohammadi, H.; Abnosi, M.H.; Hosseinzadeh, A.; Erfantalab, M. Synthesis, biological and computational study of new Schiff base hydrazones bearing 3-(4-pyridine)-5-mercapto-1,2,4-triazole moiety. Spectrochim. Acta A 2008, 71, 1474–1480. [Google Scholar] [CrossRef]
- Mostafa, Y.A.H.; Hussein, M.A.; Radwan, A.A.; Kfafy, A. Synthesis and antimicrobial activity of certain new 1,2,4-triazolo1,5-α pyrimidine derivatives. Arch. Pharm. Res. 2008, 31, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Badwaik, V.B.; Aswar, A.S. Synthesis, characterization, and biological studies of some Schiff base complexes. Russ. J. Coord. Chem. 2007, 33, 755–760. [Google Scholar] [CrossRef]
- Soni, B.; Ranawat, M.S.; Sharma, R.; Bhandari, A.; Sharma, S. Synthesis and evaluation of some new benzothiazole derivatives as potential antimicrobial agents. Eur. J. Med. Chem. 2010, 45, 2938–2942. [Google Scholar] [CrossRef] [PubMed]
- Cansiz, A.; Koparir, M.; Demirdag, A. Synthesis of some new 4,5-substituted-4H-1,2,4-triazole-3-thiol derivatives. Molecules 2004, 9, 204–212. [Google Scholar] [CrossRef] [PubMed]
- Fulias, A.; Ledeti, I.; Vlase, G.; Vlase, T. Physico-chemical solid-state characterization of pharmaceutical pyrazolones: An unexpected thermal behaviour. J. Pharm. Biomed. 2013, 81, 44–49. [Google Scholar] [CrossRef]
- Fulias, A.; Vlase, G.; Grigorie, C.; Ledeti, I.; Albu, P.; Bilanin, M.; Vlase, T. Thermal behaviour studies of procaine and benzocaine: Part 1: Kinetic analysis of the active substances under non-isothermal conditions. J. Therm. Anal. Calorim. 2013, 113, 265–271. [Google Scholar] [CrossRef]
- Ledeti, I.; Simu, G.; Vlase, G.; Săvoiu, G.; Vlase, T.; Suta, L.M.; Popoiu, C.; Fulias, A. Synthesis and solid-state characterization of Zn(II) metal complex with acetaminophen. Rev. Chim. Buchar. 2013, 64, 1127–1130. [Google Scholar]
- Ledeti, I.; Fulias, A.; Vlase, G.; Vlase, T.; Bercean, V.; Doca, N. Thermal behaviour and kinetic study of some triazoles as potential anti-inflammatory agents. J. Therm. Anal. Calorim. 2013, 114, 1295–1305. [Google Scholar] [CrossRef]
- Fulias, A.; Vlase, G.; Vlase, T.; Soica, C.; Heghes, A.; Craina, M.; Ledeti, I. Comparative kinetic analysis on thermal degradation of some cephalosporins using TG and DSC data. Chem. Cent. J. 2013, 7, 70. [Google Scholar] [CrossRef] [PubMed]
- Tita, B.; Fulias, A.; Bandur, G.; Ledeti, I.; Tita, D. Application of thermal analysis to study the compatibility of sodium diclofenac with different pharmaceutical excipients. Rev. Chim. Buchar. 2011, 62, 443–454. [Google Scholar]
- Fulias, A.; Tita, B.; Tita, D. Thermal decomposition of some benzodiazepines under non-isothermal conditions: Kinetic study. Rev. Chim. Buchar. 2009, 60, 1079–1083. [Google Scholar]
- Serra, R.; Nomen, R.; Sempere, J. The non-parametric kinetics: A new method for the kinetic study of thermoanalytical data. J. Therm. Anal. Calorim. 1998, 52, 933–943. [Google Scholar] [CrossRef]
- Serra, R.; Sempere, J.; Nomen, R. A new method for the kinetic study of thermoanalytical data: The non-parametric kinetics method. Thermochim. Acta 1998, 316, 37–45. [Google Scholar] [CrossRef]
- Vlase, T.; Vlase, G.; Doca, N.; Ilia, G.; Fuliaş, A. Coupled thermogravimetric-IR techniques and kinetic analysis by non-isothermal decomposition of Cd2+ and Co2+ vinyl-phosphonates. J. Therm. Anal. Calorim. 2009, 97, 467–472. [Google Scholar] [CrossRef]
- Vlase, T.; Vlase, G.; Birta, N.; Doca, N. Comparative results of kinetic data obtained with different methods for complex decomposition steps. J. Therm. Anal. Calorim. 2007, 88, 631–635. [Google Scholar] [CrossRef]
- Vlase, T.; Vlase, G.; Doca, N.; Bolcu, C. Processing of non-isothermal TG data: Comparative kinetic analysis with NPK method. J. Therm. Anal. Calorim. 2005, 80, 59–64. [Google Scholar] [CrossRef]
- Ozawa, T. A new method of analyzing thermogravimetric data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef]
- Flynn, J.H.; Wall, L.A. A quick direct method for determination of activation energy from thermogravimetric data. J. Polym. Sci. B 1966, 4, 323–328. [Google Scholar] [CrossRef]
- Doyle, C.D. Estimating isothermal life from thermogravimetric data. J. Appl. Sci. 1962, 6, 639–646. [Google Scholar] [CrossRef]
- Friedman, H.L. New methods for evaluating kinetic parameters from thermal analysis data. J. Polym. Sci. 1965, 6, 183–187. [Google Scholar]
- Kissinger, H.E. Reaction kinetics in differential thermal analysis. Anal. Chem. 1957, 29, 1702–1706. [Google Scholar] [CrossRef]
- Akahira, T.; Sunose, T. Joint convention of four electrical institutes. Res. Rep. Chiba Inst. Technol. 1971, 16, 22–31. [Google Scholar]
- Murray, P.; White, J. Kinetics of thermal dehydration of clays: Part IV: Interpretation of differential thermal analysis of the clay mineral. Trans. Br. Ceram. Soc. 1955, 54, 204–238. [Google Scholar]
- Wall, M.E.; Rechtsteiner, A.; Rocha, L.M. Singular value decomposition and principal component analysis. In A Practical Approach to Microarray Data Analysis; Berrar, D.P., Dubitzky, W., Granzow, M., Eds.; Kluwer Academic Publishers: Norwell, MA, USA, 2003; pp. 91–109. [Google Scholar]
- Šesták, J.; Berggren, G. Study of the kinetics of the mechanism of solid-state reactions at increasing temperatures. Thermochim. Acta 1971, 3, 1–12. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ledeţi, I.; Alexa, A.; Bercean, V.; Vlase, G.; Vlase, T.; Şuta, L.-M.; Fuliaş, A. Synthesis and Degradation of Schiff Bases Containing Heterocyclic Pharmacophore. Int. J. Mol. Sci. 2015, 16, 1711-1727. https://doi.org/10.3390/ijms16011711
Ledeţi I, Alexa A, Bercean V, Vlase G, Vlase T, Şuta L-M, Fuliaş A. Synthesis and Degradation of Schiff Bases Containing Heterocyclic Pharmacophore. International Journal of Molecular Sciences. 2015; 16(1):1711-1727. https://doi.org/10.3390/ijms16011711
Chicago/Turabian StyleLedeţi, Ionuţ, Anda Alexa, Vasile Bercean, Gabriela Vlase, Titus Vlase, Lenuţa-Maria Şuta, and Adriana Fuliaş. 2015. "Synthesis and Degradation of Schiff Bases Containing Heterocyclic Pharmacophore" International Journal of Molecular Sciences 16, no. 1: 1711-1727. https://doi.org/10.3390/ijms16011711
APA StyleLedeţi, I., Alexa, A., Bercean, V., Vlase, G., Vlase, T., Şuta, L.-M., & Fuliaş, A. (2015). Synthesis and Degradation of Schiff Bases Containing Heterocyclic Pharmacophore. International Journal of Molecular Sciences, 16(1), 1711-1727. https://doi.org/10.3390/ijms16011711









