2.1. Profiles of Biomass Growth and Chlorophyll a
N deficient algal cells proceed to a photosynthetic active status after being resupplied with nitrate, via the process termed “regreening”, which is characterized by a reassembling of the photosynthetic apparatus, re-synthesizing protein, and by utilization of Poly-P, carbohydrates, and lipids. The regreening and continual degreening processes were investigated with respect to the change of biomass and chlorophyll
a content. The time-course profiles of biomass in the three media are shown in
Figure 1A. The biomass concentration persistently increased in the media with an extracellular N source supply. The maximum biomass of C.
vulgaris obtained in the N and P condition was approximately 1160 mg·L
−1, followed by the N and P− condition with a biomass concentration of 965 mg·L
−1 after 13 days cultivation. Moreover, the biomass growth rates of N and P and N and P− during the first three days were very similar. However, there was a detrimental effect for algal cells during the continuous N deficient phase. Clearly, biomass increased slowly and the maximum concentration was obtained at the eighth day in the N− and P− condition. The increased biomass may be explained by the accumulation of lipid in algal cells with N starvation stimulation. The culture media were taken regularly for the determination of chlorophyll
a. As shown in
Figure 1B, chlorophyll
a content in the N− and P− condition dropped from 0.62% to 0.11%, and a similar decrease also occurred in the N and P− condition after a dramatic increase in the initial three days, while chlorophyll
a content maintained almost unchanged in the N and P condition (approximately at the level of 2.5%) in the latter stage of cultivation.
The optimal conditions for the regreening process are supposed to be offering not only sufficient N but also sufficient P, that is, the N and P condition in this study. It was clear that the regreening process for
C. vulgaris was completed within 3–5 days, as judged by the change of chlorophyll
a content which reached its maximum value on the fifth day. However, upon exposure to a N containing medium, cells of
C. fusca began the regreening period, with chlorophyll synthesis completed within 24 h [
13]. The content of chlorophyll
a in
Phaeodactylum tricornutum was increased in the third days after the resupply of nitrate [
14]. These different lengths of the regreening period are probably due to the different algal species.
Cell metabolism is induced to change under conditions of N starvation, and the most notable of these changes is the reassembling of the photosynthetic apparatus and the loss of photosynthetic capacity to a level even below that of spores [
12]. However, it was obvious that the biomass continually increased after external N was deficient in N− and P− conditions as shown in
Figure 1A. This phenomenon is hypothetically supported by the presumption that chlorophyll
a serves as an intracellular N source when the external N sources are exhausted [
4]. Clearly, chlorophyll
a decreased from 0.62% to 0.11% in the N− and P− condition (
Figure 1B). The phenomenon of chlorophyll
a degradation in the external N unavailable condition was also verified by other researchers. Lv
et al. [
15] reported that chlorophyll
a content of
C. vulgaris began to decrease dramatically once nitrate was completely consumed. In another algal species,
Rhodomonas sp., the content of chlorophyll
a increased from 0.5 to 1.5 pg/cell in the first N available stage and then decreased to 0.3 pg/cell when the nitrate was exhausted [
16]. However, when N−sufficient conditions returned, chlorophyll
a was again accumulated with the content increasing at a very rapid rate. The chlorophyll
a content increased obviously from 0.8% to 2.5% during the first 5 days in our experiment and then remained at its maximal level in the N and P conditions, while it dropped sharply at the later stage of N and P−. Revealing the reason for this phenomenon should get to the root of phosphate deficiency.
Figure 1.
Profiles of (A) biomass growth and (B) chlorophyll a content of C. vulgaris in different media. Values shown are averages of two samples ± range.
Figure 1.
Profiles of (A) biomass growth and (B) chlorophyll a content of C. vulgaris in different media. Values shown are averages of two samples ± range.
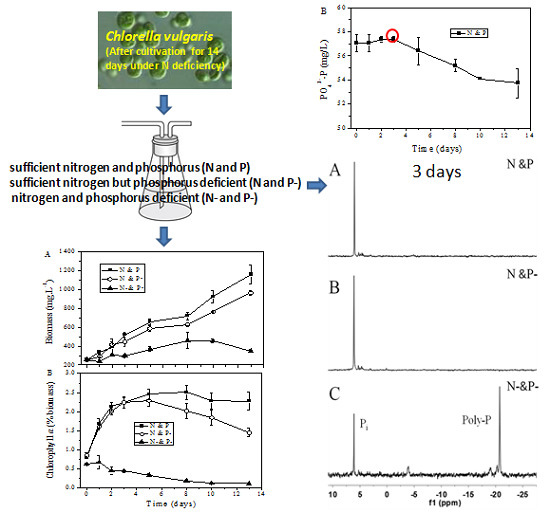
2.2. Nutrient Assimilation Profile
Figure 2 shows the time-course profiles of the concentration of N (PO
43−-N) and P (PO
43−-P) in different cultivation conditions. As shown in
Figure 2A, in the first cultivation stage, the N uptake rate was quite similar in N and P and N and P− conditions, while it gradually slowed down five days later in the N and P− condition.
Figure 2B shows the orthophosphate assimilation profile in the N and P condition. No obvious consumption was observed during the first stage of cultivation, but PO
43−-P began to be assimilated after three days cultivation (
Figure 2B).
Figure 2.
Profiles of (A) nitrogen (NO3−-N) and (B) orthophosphate (PO43−-P) assimilation by C. vulgaris cultured in different conditions. Values shown are averages of two samples ± range
Figure 2.
Profiles of (A) nitrogen (NO3−-N) and (B) orthophosphate (PO43−-P) assimilation by C. vulgaris cultured in different conditions. Values shown are averages of two samples ± range
2.3. Changes in Storage Products during the Regreening Process
To investigate the fate of intracellular Poly-P with respect to the variation of lipid productivity during the regreening process, changes in the contents of the lipids, starch, and protein of
C. vulgaris over time were determined. As shown in
Figure 3A, lipid content decreased slightly in the first day and then increased dramatically from 26.3% to 37.4% in the N− and P− condition, which was in the continual degreening process with increased lipid content. The possible reason for the slight decrease of lipid content in the first day is probably due to self-regulating for the new cultivation condition or can be explained by systematic errors. However, in the regreening process, lipid content decreased rapidly from 39.3% to an average level of 15.8% within 24 hours upon supplying with N, and the low level of 15.8% was the usual lipid content in normal growing cells of
C. vulgaris [
7]. As shown in
Figure 3B,C, protein and starch contents remained almost unchanged in N− and P−, which can be explained by the fact that cells were still in the exoteric N depletion stress condition. In terms of the regreening process in N and P and N and P−, the changes of starch and protein contents all lagged behind lipid variation. Starch content decreased rapidly within 5 days and then remained at low levels during the following 8 days with final contents of 5.0% and 8.3% in N and P and N and P−, respectively (
Figure 3B). However, the protein content increased dramatically to the normal growth level within 5 days, and the final contents in the N and P and N and P− conditions were 51.3% and 48.8%, respectively.
Figure 3.
Storage products of C. vulgaris grown in different media. (A) lipid; (B) starch; and (C) protein. Values shown are averages of two samples ± range.
Figure 3.
Storage products of C. vulgaris grown in different media. (A) lipid; (B) starch; and (C) protein. Values shown are averages of two samples ± range.
2.4. Microscopic Analysis of Lipid Droplets and Poly-P and 31P NMR
C. vulgaris cells change their metabolic pathways to accumulate a large amount of neutral lipids and Poly-P granules upon stimulation by N starvation [
7]. If the cells are suspended in different nutrient conditions, changing profiles of neutral lipids and Poly-P can be monitored by staining with Nile Red and DAPI. As shown in
Figure 4, the gold-yellow fluorescence observed indicated that a high content of neutral lipids still existed in cells under the N− and P− condition, while the lipid contents were rather low in N and P and N and P− conditions after one day cultivation. From the DAPI staining images, the bright yellow fluorescence in the initial cells and the cells under N− and P− condition illustrated the existence of high Poly-P content, while it was hardly seen in the three-day cultivation cells under N and P and N and P− conditions. Meanwhile, red fluorescence coming from the chlorophyll
a indicated that cells gradually accumulated a certain amount of chlorophyll
a (
Figure 4).
Figure 4.
Observation of neutral lipid and Poly-P using Nile Red and DAPI staining. Above: The golden yellow fluorescence in the images indicates the presence of neutral lipid, and the red fluorescence is the autofluorescence of chlorophyll. Below: Blue fluorescence comes from nucleus staining, bright yellow fluorescence shows the Poly-P, and red fluorescence comes from chlorophyll.
Figure 4.
Observation of neutral lipid and Poly-P using Nile Red and DAPI staining. Above: The golden yellow fluorescence in the images indicates the presence of neutral lipid, and the red fluorescence is the autofluorescence of chlorophyll. Below: Blue fluorescence comes from nucleus staining, bright yellow fluorescence shows the Poly-P, and red fluorescence comes from chlorophyll.
Figure 5 shows NMR spectra of
C. vulgaris in N and P (A), N and P− (B), and N− and P− (C) conditions after three-day cultivation. The intense signal of the mid-chain P groups of Poly-P at −21 ppm illustrated that the algal cells at N− and P− still had high amounts of Poly-P, while the distinct peak at approximately 6.5 ppm showed that inorganic orthophosphate were very abundant in the cells of the three cultivation conditions.
Poly-P, judged by its structure, can serve as a phosphate/energy source and plays a significant role during the regreening process where the cells recover when the external conditions once again favor cell growth [
12]. The intense signal of the mid-chain P groups of Poly-P shown in
Figure 5C indicated that, as compared with N resupply conditions, Poly-P could hardly be utilized smoothly in N− and P− conditions. This phenomenon leads to the conclusion that the prerequisite for Poly-P usage is the existence of N, since Poly-P is also proven as a regulator for stress and survival [
10]. Nevertheless, Poly-P could almost not be found in cells in the N and P and N and P− conditions after 3 days cultivation based on the signals shown in
31P NMR (
Figure 5A,B) and DAPI staining images (
Figure 4). The data presented in
Figure 2B show that orthophosphate was not assimilated until the fourth day in the N and P condition. It therefore seemed more reasonable to assume that Poly-P was obligatory during the first stage of regreening progress. Similarly, Kuesel
et al. [
12] noted that
C. fusca cells contained the same amount of Poly-P in both phosphate supplied and unsupplied conditions after 8 h regreening, which indicated that Poly-P, as an important phosphate source, is utilized first during the first period of the regreening process.
The functioning of Poly-P as an energy and phosphate source is widely acknowledged. In many organisms, several enzymes are involved in the process to catalyze the transfer of energy and Pi among AMP, ATP and Poly-P [
9,
10]. For example, Poly-P kinase and AMP−phosphotransferase are two important enzymes necessary for the energy release from Poly-P [
10]. Polyphosphatases, the enzymes catalyzing the hydrolysis of Poly-P to Pi, are responsible for offering a phosphate source via Poly-P [
9,
17].
Figure 5.
The 31P NMR spectra of C. vulgaris cultured in N and P (A); N and P− (B); and N− and P− (C) media for three days. Pi; inorganic phosphate, Poly-P; polyphosphates.
Figure 5.
The 31P NMR spectra of C. vulgaris cultured in N and P (A); N and P− (B); and N− and P− (C) media for three days. Pi; inorganic phosphate, Poly-P; polyphosphates.
2.5. Productivity of FAMEs
Productivity of FAMEs is an important indicator of biodiesel producing capacity, and can be calculated from the FAME content and biomass productivity. The time-course profiles of FAME productivity in the three culture conditions are shown in
Figure 6. FAME productivity increased gradually during the degreening and regreening processes. Relatively higher FAME productivity was observed in the N− and P− culture and the highest was 10.7 mg·L
−1·day
−1 at day 9, which was attributed to the higher FAME content. In the regreening process, regarding the effect of external P, the slightly higher FAME productivity in the N and P as compared with the N and P− cultures indicated the positive effect of P on biodiesel production.
Figure 6.
FAME productivity of C. vulgaris cultured in N and P, N and P−, and N− and P− media. Values shown are averages of two samples ± range.
Figure 6.
FAME productivity of C. vulgaris cultured in N and P, N and P−, and N− and P− media. Values shown are averages of two samples ± range.
2.6. Overall Analysis of this Work
The fact that the rapid consumption of Poly-P in N and P and N and P− conditions indicated that Poly-P could only satisfy the needs of regreening cells for a very short time and, without external phosphate replenishment, the algal cells would be in a state of P depletion. The nitrate assimilation profile in
Figure 2A clearly showed that the process of N consumption was strictly influenced by P starvation in N and P− conditions after 5 days of cultivation. Then, algal cells in this condition gradually entered another state with the simultaneous absence of N and P. Chlorophyll
a, an N− rich compound, was then degraded to offer endogenous N as shown in the N and P− condition. Without the high photosynthetic activity obtained from chlorophyll, the biomass growth lagged behind with the lower growth rate in N and P− as compared with N and P condition. Undoubtedly, in terms of the lipid reviving process (as shown in
Figure 6), FAME productivity was dramatically depressed in N and P− condition by lower biomass productivity in spite of the same lipid content in the two N sufficient conditions. These results revealed that with regard to the regreening process, especially for biomass and lipid production revival, it was necessary to supply external N and P at the same time.
The degreening process for
C. vulgaris was also studied previously [
7]. It was demonstrated that P was assimilated rapidly in the degreening process as compared with nutrients complete condition. However, the stationary phase was not reached after 14 days cultivation in N deficient condition with P sufficient supply [
7]. It is reasonable that the late stage of the growth would be N− and P− condition after P was assimilated completely in the previous study. Obviously, algae cells got into stationary phase after a slow growth stage for 8 days (
Figure 1). In this work, the difference of biomass growth rate between N and P and N and P− conditions was smaller than that in our previous study [
7]. The reason for this is probably the Poly-P existed in algae cells in N and P− condition. Moreover, the absorption of N nearly ceased in P deficiency condition in our previous study [
7], while it only stopped completely after 10 days in N and P− due to the existence of Poly-P as internal P. This phenomenon, once again, demonstrates that the existence of P is the prerequisite for nitrogen utilization for algal cells.