Microanalysis, Pharmacokinetics and Tissue Distribution of Polysaccharide-Protein Complexes from Longan Pulp in Mice
Abstract
:1. Introduction
2. Results and Discussion
2.1. Purification of Longan Polysaccharide Complexes (LPP) and Fluorescein Isothiocyanate-Labeled LPP (LPPF)


2.2. Spectral Features of LPP and LPPF
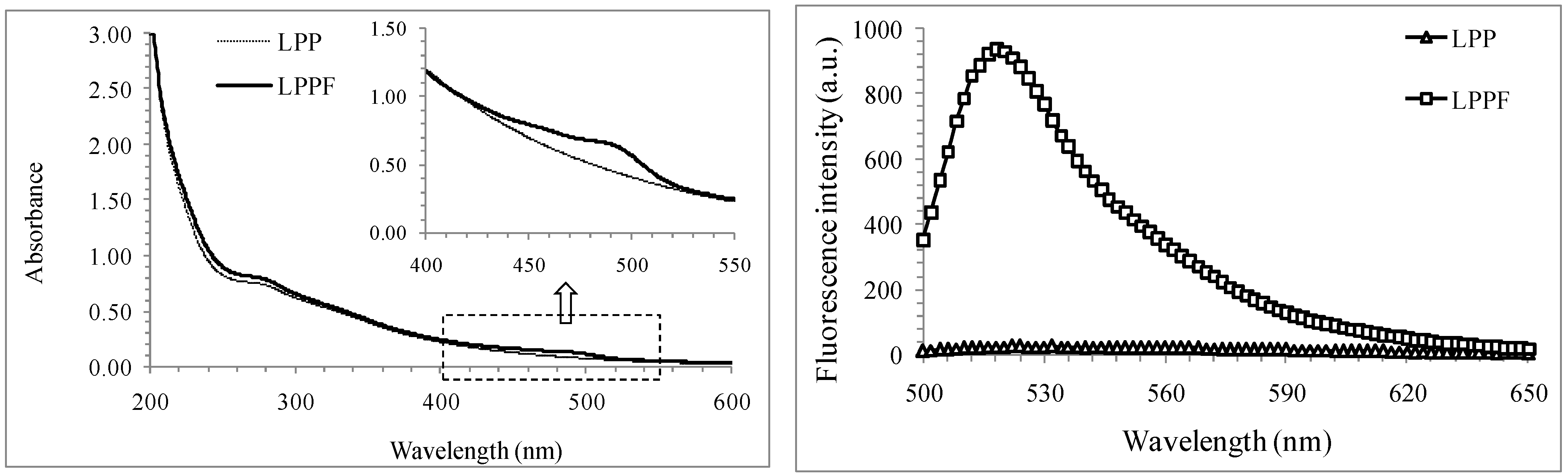
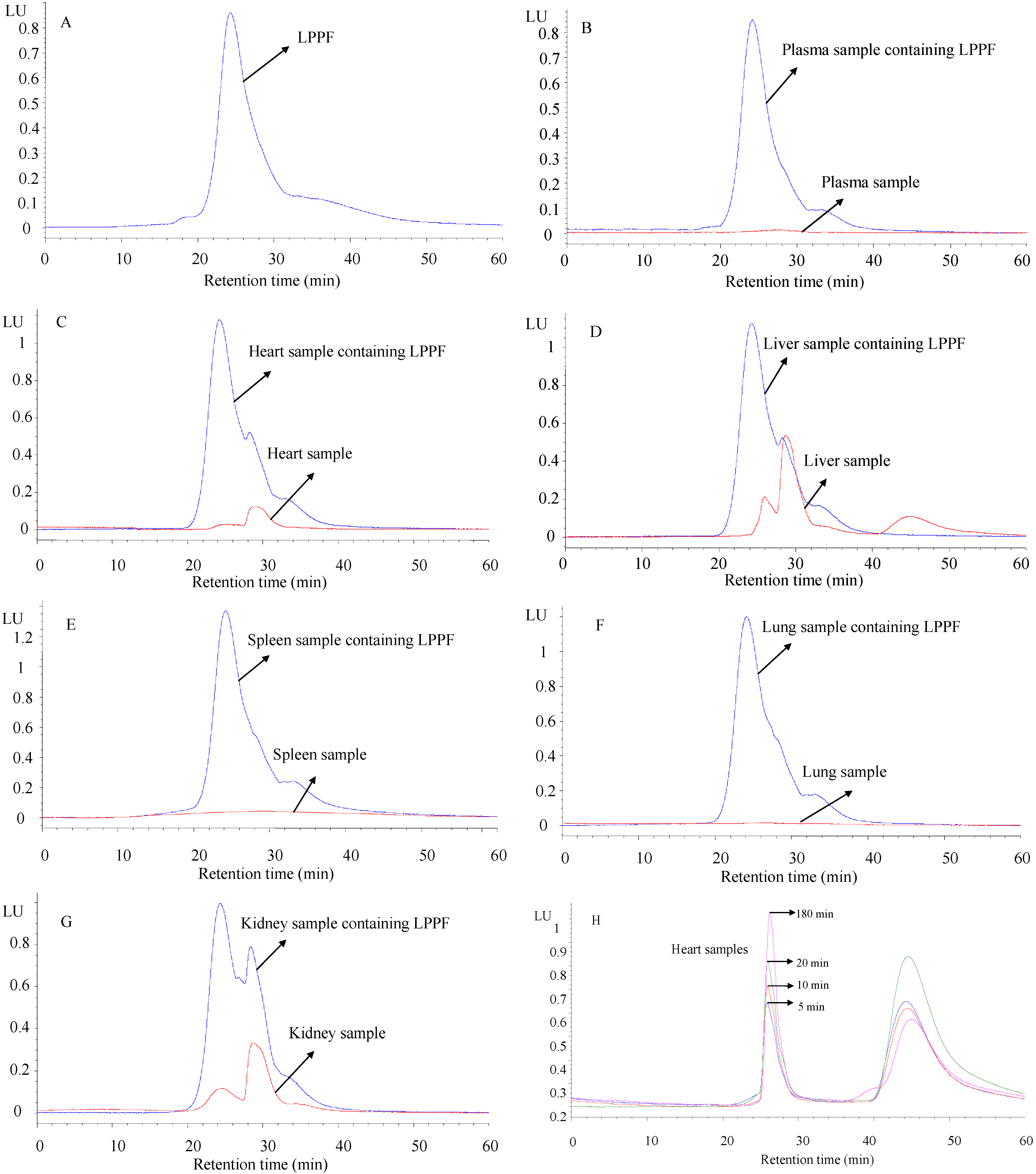
2.3. Method Validation
| Biological Samples | Standard Curves | Correlation Coefficients | Linear Ranges * (µg/mL) |
|---|---|---|---|
| Blank | y = 2.199x + 2.273 | 0.998 | 1–200 µg/mL |
| Plasma | y = 2.223x + 1.034 | 0.996 | 1–200 µg/mL |
| Spleen | y = 2.349x − 0.485 | 0.996 | 1–200 µg/mL |
| Lung | y = 2.138x + 2.616 | 0.995 | 1–200 µg/mL |
| Biological Samples | Added Concentration (µg/mL) | Relative Recovery (%) | Precision (RSD, %) | |
|---|---|---|---|---|
| Intra-Day | Inter-Day | |||
| Blank | 2 | 99.4 ± 4.3 | 3.4 | 4.0 |
| 40 | 101.7 ± 3.0 | 2.4 | 2.9 | |
| 80 | 101.4 ± 2.3 | 3.1 | 4.2 | |
| Plasma | 2 | 98.2 ± 5.1 | 4.5 | 4.5 |
| 40 | 102.3 ± 4.1 | 3.2 | 2.9 | |
| 80 | 103.4 ± 5.2 | 5.1 | 2.6 | |
| Spleen | 2 | 106.4 ± 3.9 | 3.6 | 3.5 |
| 40 | 96.7 ± 5.7 | 4.4 | 5.0 | |
| 80 | 105.4 ± 3.3 | 5.7 | 3.5 | |
| Lung | 2 | 93.7 ± 2.9 | 3.0 | 4.2 |
| 40 | 104.2 ± 3.1 | 4.4 | 3.8 | |
| 80 | 96.5 ± 3.4 | 2.5 | 6.9 | |
2.4. Pharmacokinetics and Tissue Distribution of LPPF

| Parameters * | Values |
|---|---|
| Half-life of distribution phase t1/2α (min) | 2.23 ± 0.35 |
| Half-life of elimination phase t1/2β (min) | 39.11 ± 2.73 |
| Apparent volume of distribution of the central compartment V1 (L/kg) | 0.09 ± 0.01 |
| Apparent volume of distribution of the peripheral compartment V2 (L/kg) | 0.26 ± 0.03 |
| Clearance of the central compartment CL1 (L/h/kg) | 0.53 ± 0.03 |
| Clearance of the peripheral compartment CL2 (L/h/kg) | 0.60 ± 0.05 |
| Area under concentration–time curve AUC0–t (mg·h/L) | 56.80 ± 3.44 |
| Area under concentration–time curve AUC0–∞ (mg·h/L) | 58.85 ± 3.51 |
| Mean retention time MRT0–t (h) | 1.15 ± 0.04 |
| Mean retention time MRT0–∞ (h) | 1.39 ± 0.08 |
3. Experimental Section
3.1. Materials
3.2. Animals and Biological Sample Preparation
3.3. Polysaccharide–Protein Complex Preparation
3.4. Spectrum Analysis
3.5. HPSEC Analysis
3.5.1. Detection Conditions
3.5.2. Selectivity
3.5.3. Quantitative Determination
3.5.4. Precision and Accuracy
3.6. Animal Experiment
3.7. Data Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Park, S.J.; Park, D.H.; Kim, D.H.; Lee, S.; Yoon, B.H.; Jung, W.Y.; Lee, K.T.; Cheong, J.H.; Ryu, J.H. The memory-enhancing effects of Euphoria longan fruit extract in mice. J. Ethnopharmacol. 2010, 128, 160–165. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; He, N.; Ling, X.; Ye, M.; Zhang, C.; Shao, W.; Yao, C.; Wang, Z.; Li, Q. The isolation and characterization of polysaccharides from longan pulp. Sep. Purif. Technol. 2008, 63, 226–230. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q. Optimization of ultrasonic extraction of polysaccharides from dried longan pulp using response surface methodology. Carbohydr. Polym. 2010, 80, 19–25. [Google Scholar] [CrossRef]
- Zhong, K.; Wang, Q.; He, Y.; He, X. Evaluation of radicals scavenging, immunity-modulatory and antitumor activities of longan polysaccharides with ultrasonic extraction on in S180 tumor mice models. Int. J. Biol. Macromol. 2010, 47, 356–360. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Jiang, Y.; Lin, S.; Wen, L.; Wu, D.; Zhao, M.; Chen, F.; Jia, Y.; Yang, B. Structural identification of (1→6)-α-d-glucan, a key responsible for the health benefits of longan, and evaluation of anticancer activity. Biomacromolecules 2013, 14, 1999–2003. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Zhang, M.W.; Liao, S.T.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C.; Tang, X.J.; Zhang, Y. Structural features and immunomodulatory activities of polysaccharides of longan pulp. Carbohydr. Polym. 2012, 87, 636–643. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, M.W.; Liao, S.T.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C.; Tang, X.J.; Zhang, Y. Effects of alkali dissociation on the molecular conformation and immunomodulatory activity of longan pulp polysaccharide (LPI). Carbohydr. Polym. 2012, 87, 1311–1317. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Zhang, R.F.; Deng, Y.Y.; Yang, B.; Wei, Z.C. Immunomodulatory activity of polysaccharide-protein complex of longan (Dimocarpus longan lour.) pulp. Molecules 2011, 16, 10324–10336. [Google Scholar] [CrossRef] [PubMed]
- Kaneo, Y.; Ueno, T.; Tanaka, T.; Iwase, H.; Yamaguchi, Y.; Uemura, T. Pharmacokinetics and biodisposition of fluorescein-labeled arabinogalactan in rats. Int. J.Pharm. 2000, 201, 59–69. [Google Scholar] [CrossRef]
- Yi, Y.; Wang, H.-X.; He, J.-R. Research progresses of pharmacokinetics of polysaccharides. Acta Pharm. Sin. 2014, 49, 443–449. [Google Scholar]
- Lin, X.; Wang, Z.; Sun, G.; Shen, L.; Xu, D.; Feng, Y. A sensitive and specific HPGPC-FD method for the study of pharmacokinetics and tissue distribution of Radix Ophiopogonis polysaccharide in rats. Biomed. Chromatogr. 2010, 24, 820–825. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y. Label of Biomacromolecular Drugs and Their Pharmacokinetic Studies. Ph.D. Thesis, The Second Military Medical University, Shanghai, China, 2010. [Google Scholar]
- Balogh, L.; Polyak, A.; Mathe, D.; Kiraly, R.; Thuroczy, J.; Terez, M.; Janoki, G.; Ting, Y.; Bucci, L.R.; Schauss, A.G. Absorption, uptake and tissue affinity of high-molecular-weight hyaluronan after oral administration in rats and dogs. J. Agric. Food Chem. 2008, 56, 10582–10593. [Google Scholar] [CrossRef] [PubMed]
- Lendvai, N.; Casadevall, A.; Liang, Z.; Goldman, D.L.; Mukherjee, J.; Zuckier, L. Effect of immune mechanisms on the pharmacokinetics and organ distribution of cryptococcal polysaccharide. J. Infect. Dis. 1998, 177, 1647–1659. [Google Scholar] [CrossRef] [PubMed]
- De Lima, C.R.; Baccarin, R.Y.A.; Michelacci, Y.M. Reliability of 1,9-dimethylmethylene blue tests in comparison to agarose gel electrophoresis for quantification of urinary glycosaminoglycans. Clin. Chim. Acta 2007, 378, 206–215. [Google Scholar] [CrossRef]
- Guimarães, M.A.M.; Mourão, P.A.S. Urinary excretion of sulfated polysaccharides administered to wistar rats suggests a renal permselectivity to these polymers based on molecular size. Biochim. Biophys. Acta 1997, 1335, 161–172. [Google Scholar] [CrossRef]
- Ricea, P.J.; Lockharta, B.E.; Barkerb, L.A.; Adamsb, E.L.; Ensleyc, H.E.; Williams, D.L. Pharmacokinetics of fungal (1–3)-β-d-glucans following intravenous administration in rats. Int. Immunopharmacol. 2004, 4, 1209–1215. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Han, B.; Shao, K.; Yang, Z.; Peng, Y.; Yang, Y.; Liu, W. Effects of molecular weights on the absorption, distribution and urinary excretion of intraperitoneally administrated carboxymethyl chitosan in rats. J. Mater. Sci. 2012, 12, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Roth, R.I.; Grunfeld, C.; Feingold, K.R.; Levin, J. Soluble (1→3)-β-d-glucan purified from Candida albicans: Biologic effects and distribution in blood and organs in rabbits. J. Lab. Clin. Med. 1996, 128, 103–114. [Google Scholar] [CrossRef]
- Yi, Y.; Liao, S.T.; Zhang, M.W.; Shi, J.; Zhang, R.F.; Deng, Y.Y.; Wei, Z.C. Physicochemical characteristics and immunomodulatory activities of three polysaccharide-protein complexes of longan pulp. Molecules 2011, 16, 6148–6164. [Google Scholar] [CrossRef] [PubMed]
- He, L.; Ji, P.; Cheng, J.; Wang, Y.; Qian, H.; Li, W.; Gong, X.; Wang, Z. Structural characterization and immunostimulatory activity of a novel protein-bound polysaccharide produced by Hirsutella sinensis Liu, Guo, Yu & zeng. Food Chem. 2013, 141, 946–953. [Google Scholar]
- Lv, Z.H. Studies on Fluorescent Labeling and Pharmacokinetics of Marine Sufated Polysaccharide PS916. Ph.D. Thesis, Ocean University of China, Qindao, China, 2008. [Google Scholar]
- Zeng, L.; Qin, C.; Wang, W.; Chi, W.; Li, W. Absorption and distribution of chitosan in mice after oral administration. Carbohydr. Polym. 2008, 71, 435–440. [Google Scholar] [CrossRef]
- Xu, D.-S.; Feng, Y.; Lin, X.; Deng, H.-L.; Fang, J.-N.; Dong, Q. Isolation, purification and structural analysis of a polysaccharide MDG-1 from Ophiopogon japonicus. Acta Pharm. Sin. 2005, 40, 636–639. [Google Scholar]
- Wei, X.; Xi, X.; Wu, M.; Wang, Y. A novel method for quantitative determination of tea polysaccharide by resonance light scattering. Spectrochim. Acta Part A 2011, 79, 928–933. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Studies on Pharmacokinetics and Tissue Distribution of Lentinan Liposome. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2010. [Google Scholar]
- Cai, Y. Studies on Pharmacodynamics and Pharmacokinetics of The lentinan Liposome. Ph.D. Thesis, Huazhong University of Science and Technology, Wuhan, China, 2009. [Google Scholar]
- Haag, R.; Kratz, F. Polymer therapeutics: Concepts and applications. Angew. Chem. Int. Ed. 2006, 45, 1198–1215. [Google Scholar] [CrossRef] [PubMed]
- Peng, F. Pharmacokinetics Research on Bletillan Injections. Ph.D. Thesis, Soochow University, Suzhou, China, 2010. [Google Scholar]
- Lin, X.; Wang, Z.-J.; Huang, F.; Liang, S.; Shen, L.; Feng, Y.; Ruan, K.-F. Long-circulating delivery of bioactive polysaccharide from Radix Ophiopogonis by pegylation. Int. J. Nanomed. 2011, 6, 2865–2872. [Google Scholar] [CrossRef] [PubMed]
- Lin, X.; Wang, Z.-J.; Wang, S.; Shen, L.; Feng, Y.; Ruan, K.-F.; Xu, D.-S. Comparison of tissue distribution of a pegylated Radix Ophiopogonis polysaccharide in mice with normal and ischemic myocardium. Eur. J. Pharm. Biopharm. 2011, 79, 621–626. [Google Scholar] [CrossRef] [PubMed]
- Kaneo, Y.; Uemura, T.; Tanaka, T.; Kanoh, S. Polysaccharides as drug carriers: Biodisposition of fluorescein-labeled dextrans in mice. Biol. Pharm. Bull. 1997, 20, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Prescott, J.H.; Enriquez, P.; Jung, C.; Menz, E.; Groman, E.V. Larch arabinogalactan for hepatic drug delivery: Isolation and characterization of a 9 kDa arabinogalactan fragment. Carbohydr. Res. 1995, 278, 113–128. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Pebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Skidana, I.; Grunwaldb, J.; Thekkedatha, R.; Degterevc, A.; Torchilina, V. A HPLC method for the quantitative determination of N-(2-hydroxy-5-nitrophenylcarbamothioyl)-3,5-dimethylbenzamide inbiological samples. J. Chromatogr. B 2011, 879, 1610–1616. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, T.; Sun, J.; Yi, Y.; Wang, H.-X.; Hang, F.; Ai, Y.-W.; Wang, L.-M. Microanalysis, Pharmacokinetics and Tissue Distribution of Polysaccharide-Protein Complexes from Longan Pulp in Mice. Int. J. Mol. Sci. 2015, 16, 24403-24416. https://doi.org/10.3390/ijms161024403
Min T, Sun J, Yi Y, Wang H-X, Hang F, Ai Y-W, Wang L-M. Microanalysis, Pharmacokinetics and Tissue Distribution of Polysaccharide-Protein Complexes from Longan Pulp in Mice. International Journal of Molecular Sciences. 2015; 16(10):24403-24416. https://doi.org/10.3390/ijms161024403
Chicago/Turabian StyleMin, Ting, Jie Sun, Yang Yi, Hong-Xun Wang, Fei Hang, You-Wei Ai, and Li-Mei Wang. 2015. "Microanalysis, Pharmacokinetics and Tissue Distribution of Polysaccharide-Protein Complexes from Longan Pulp in Mice" International Journal of Molecular Sciences 16, no. 10: 24403-24416. https://doi.org/10.3390/ijms161024403






