Comparison of Simple Eudragit Microparticles Loaded with Prednisolone and Eudragit-Coated Chitosan-Succinyl-Prednisolone Conjugate Microparticles: Part II. In Vivo Evaluation of Efficacy, Toxicity, and Biodisposition Characteristics
Abstract
:1. Introduction
2. Results and Discussion
2.1. Particle Characteristics of ES-MP, Ch-MP, and Ch-MP/ES

| Microparticles | PD Content (%, w/w) | Particle Size (µm) |
|---|---|---|
| ES-MP | 3.73 ± 0.74 | 1.2 ± 0.3 |
| Ch-MP | 5.44 ± 0.17 | 2.8 ± 2.2 |
| Ch-MP/ES | 2.27 ± 0.27 | 18.7 ± 12.3 |
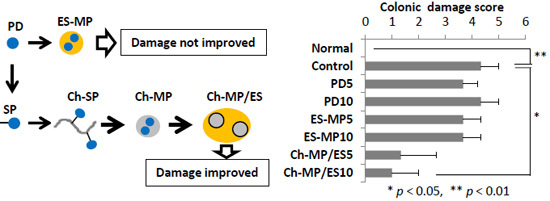
2.2. Evaluation of Efficacy and Toxicity


2.3. Gastrointestinal Drug Distribution

2.4. Plasma Concentration–Time Profiles

3. Experimental Section
3.1. Materials
3.2. Animals
3.3. Preparation of ES-MP
3.4. Preparation of Ch-MP/ES
3.5. Characterization of Microparticles
3.6. In Vivo Examination of Efficacy and Toxicity

3.7. Investigation of Gastrointestinal Drug Distribution
3.8. Examination of Plasma Concentrations–Time Profiles
3.9. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Torres, E.A.; de Jesús, R.; Pérez, C.M.; Iñesta, M.; Torres, D.; Morell, C.; Just, E. Prevalence of inflammatory bowel disease in an insured population in Puerto Rico during 1996. P. R. Health Sci. J. 2003, 22, 253–258. [Google Scholar] [PubMed]
- Ananthakrishnan, A.N. Epidemiology and risk factors for IBD. Nat. Rev. Gastroenterol. Hepatol. 2015, 12, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Magro, F.; Portela, F. Management of inflammatory bowel disease with infliximab and other anti-tumor necrosis factor alpha therapies. BioDrugs 2010, 24, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Marko, B.; Prka, L. Anti-TNF therapy in treatment of luminal Crohn’s disease. Acta Med. Croat. 2013, 67, 179–189. [Google Scholar]
- Hamilton, I.; Pinder, I.F.; Dickinson, R.J.; Ruddell, W.S.; Dixon, M.F.; Axon, A.T. A comparison of prednisolone enemas with low-dose oral prednisolone in the treatment of acute distal ulcerative colitis. Dis. Colon Rectum 1984, 27, 701–702. [Google Scholar] [CrossRef] [PubMed]
- Ardizzone, S.; Bianchi, P.G. A practical guide to the management of distal ulcerative colitis. Drugs 1998, 55, 519–542. [Google Scholar] [CrossRef] [PubMed]
- Love, B.L.; Miller, A.D. Extended-release mesalamine granules for ulcerative colitis. Ann. Pharmacother. 2012, 46, 1529–1536. [Google Scholar] [CrossRef] [PubMed]
- Fiegel, V.G. Compative studies of effects and side effects of hydrocortisone, prednisone, triamcinoline and dexamethasone. Arzneimittelforschung 1975, 25, 560–563. [Google Scholar] [PubMed]
- Onishi, H.; Oosegi, T.; Machida, Y. Efficacy and toxicity of Eudragit-coated chitosan-succinyl-prednisolone conjugate microspheres using rats with 2,4,6-trinitrobenzenesulfonic acid-induced colitis. Int. J. Pharm. 2008, 358, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Yano, H.; Hirayama, F.; Kamada, M.; Arima, H.; Uekama, K. Colon-specific delivery of prednisolone-appended alpha-cyclodextrin conjugate: Alleviation of systemic side effect after oral administration. J. Control. Release 2002, 79, 103–112. [Google Scholar] [CrossRef]
- Van den Hoven, J.M.; Hofkens, W.; Wauben, M.H.; Wagenaar-Hilbers, J.P.; Beijnen, J.H.; Nuijen, B.; Metselaar, J.M.; Storm, G. Optimizing the therapeutic index of liposomal glucocorticoids in experimental arthritis. Int. J. Pharm. 2011, 416, 471–477. [Google Scholar] [CrossRef] [PubMed]
- Bendas, E.R.; Christensen, J.M.; Ayres, J.W. Development and in vitro evaluation of mesalamine delayed release pellets and tableted reservoir-type pellets. Drug Dev. Ind. Pharm. 2010, 36, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Lamprecht, A.; Ubrich, N.; Yamamoto, H.; Schäfer, U.; Takeuchi, H.; Maincent, P.; Kawashima, Y.; Lehr, C.M. Biodegradable nanoparticles for targeted drug delivery in treatment of inflammatory bowel disease. J. Pharmacol. Exp. Ther. 2001, 299, 775–781. [Google Scholar] [PubMed]
- Ali, H.; Weigmann, B.; Neurath, M.F.; Collnot, E.M.; Windbergs, M.; Lehr, C.M. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J. Control. Release 2014, 183, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Kong, H.; Lee, Y.; Hong, S.; Han, J.; Choi, B.; Jung, Y.; Kim, Y.M. Sulfate-conjugated methylprednisolone as a colon-targeted methylprednisolone prodrug with improved therapeutic properties against rat colitis. J. Drug Target. 2009, 17, 450–458. [Google Scholar] [CrossRef] [PubMed]
- El-Gibaly, I. Oral delayed-release system based on Zn-pectinate gel (ZPG) microparticles as an alternative carrier to calcium pectinate beads for colonic drug delivery. Int. J. Pharm. 2002, 232, 199–211. [Google Scholar] [CrossRef]
- Onishi, H.; Kikuchi, H.; Machida, Y. Comparison of simple Eudragit microparticles loaded with prednisolone and Eudragit-coated chitosan-succinyl-prednisolone conjugate microparticles: Part I. Particle characteristics and in vitro evaluation as a colonic delivery system. Drug Dev. Ind. Pharm. 2012, 38, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Tozaki, H.; Odoriba, T.; Okada, N.; Fujita, T.; Terabe, A.; Suzuki, T.; Okabe, S.; Muranishi, S.; Yamamoto, A. Chitosan capsules for colon-specific drug delivery: Enhanced localization of 5-aminosalicylic acid in the large intestine accelerates healing of TNBS-induced colitis in rats. J. Control. Release 2002, 82, 51–61. [Google Scholar] [CrossRef]
- Morris, G.P.; Beck, P.L.; Herridge, M.S.; Depew, W.T.; Szewczuk, M.R.; Wallace, J.L. Hapten-induced model of chronic inflammation and ulceration in the rat colon. Gastroenterology 1989, 96, 795–803. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Onishi, H.; Kikuchi, H. Comparison of Simple Eudragit Microparticles Loaded with Prednisolone and Eudragit-Coated Chitosan-Succinyl-Prednisolone Conjugate Microparticles: Part II. In Vivo Evaluation of Efficacy, Toxicity, and Biodisposition Characteristics. Int. J. Mol. Sci. 2015, 16, 26125-26136. https://doi.org/10.3390/ijms161125949
Onishi H, Kikuchi H. Comparison of Simple Eudragit Microparticles Loaded with Prednisolone and Eudragit-Coated Chitosan-Succinyl-Prednisolone Conjugate Microparticles: Part II. In Vivo Evaluation of Efficacy, Toxicity, and Biodisposition Characteristics. International Journal of Molecular Sciences. 2015; 16(11):26125-26136. https://doi.org/10.3390/ijms161125949
Chicago/Turabian StyleOnishi, Hiraku, and Hisashi Kikuchi. 2015. "Comparison of Simple Eudragit Microparticles Loaded with Prednisolone and Eudragit-Coated Chitosan-Succinyl-Prednisolone Conjugate Microparticles: Part II. In Vivo Evaluation of Efficacy, Toxicity, and Biodisposition Characteristics" International Journal of Molecular Sciences 16, no. 11: 26125-26136. https://doi.org/10.3390/ijms161125949
APA StyleOnishi, H., & Kikuchi, H. (2015). Comparison of Simple Eudragit Microparticles Loaded with Prednisolone and Eudragit-Coated Chitosan-Succinyl-Prednisolone Conjugate Microparticles: Part II. In Vivo Evaluation of Efficacy, Toxicity, and Biodisposition Characteristics. International Journal of Molecular Sciences, 16(11), 26125-26136. https://doi.org/10.3390/ijms161125949