Serum Biomarkers of (Anti)Oxidant Status for Epidemiological Studies
Abstract
:1. Introduction
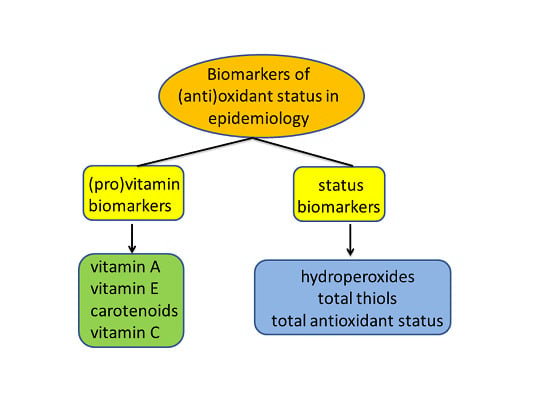
2. Biomarker Selection
3. Biomarkers of Antioxidant Vitamins and Provitamins
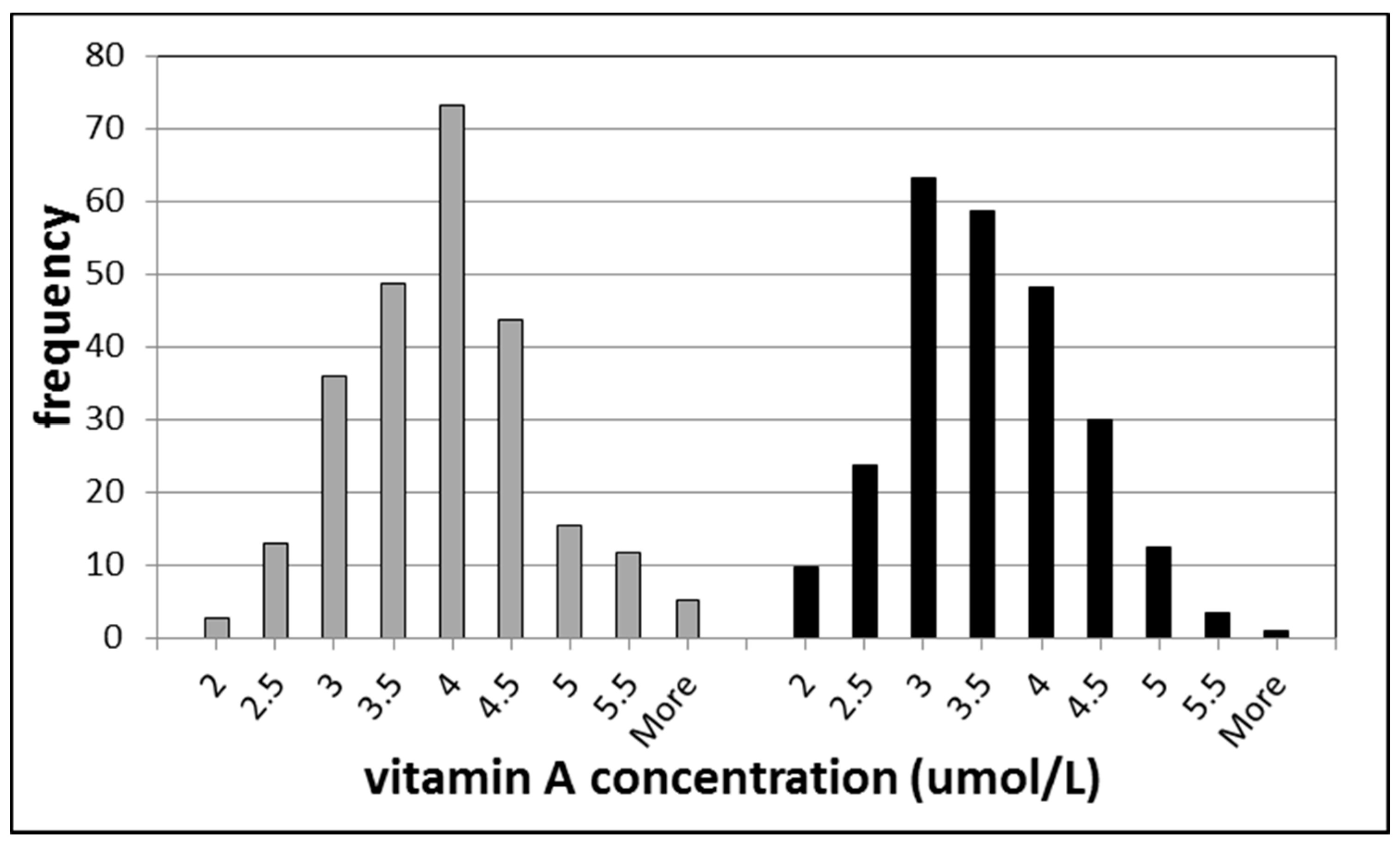
3.1. Biomarkers of Vitamin A
3.2. Biomarkers of Vitamin E

3.3. Biomarkers of Carotenoids
3.4. Biomarker of Vitamin C
3.5. Measurements of Biomarkers of Antioxidant (pro)Vitamins



4. Biomarkers of Total Antioxidant Defense
5. Biomarkers of Oxidant Status
5.1. Biomarkers of Lipid Peroxidation
5.2. Biomarkers Based on Hydroperoxide Detection
5.3. Biomarkers of Redox Status
6. Conclusions
| Biomarker | Time Range | Normal Range | Unhealthy Range | Method of Analysis | Multi-Component | Samples/Day |
|---|---|---|---|---|---|---|
| Vitamin A | weeks-months | 1.8–7.0 µmol/L [48] | no data | HPLC | yes | 80 |
| Vitamin E | weeks-months | 14–40 µmol/L [49] | <21 µmol/L [50] | HPLC | yes | 80 |
| Carotenoids | weeks-months | 50–650 µg/L [49] | no data | HPLC | yes | 80 |
| Vitamin C | hours-days | 4.1–20.0 mg/L [49] | 2.0–4.1 mg/L [49] | HPLC or Auto-Analyzer | no | 100 |
| BAP | days-months | >2200 µmol/L | <2000 µmol/L | Auto-Analyzer | yes | 240 |
| ROM | days-months | 250–300 Carr·U | >320 Carr·U | Auto-Analyzer | yes | 240 |
| TTL | days-months | no data | no data | Auto-Analyzer | yes | 240 |
| SHp | days-months | 450–650 µmol/L | <450 µmol/L | Auto-Analyzer | yes | 240 |

Acknowledgments
Author Contributions
Conflicts of Interest
References
- Sofi, F.; Abbate, R.; Gensini, G.F.; Casini, A. Accruing evidence on benefits of adherence to the Mediterranean diet on health: An updated systematic review and meta-analysis. Am. J. Clin. Nutr. 2010, 92, 1189–1196. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, L.L.; Simonetti, R.G.; Gluud, C. Antioxidant supplements for prevention of mortality in healthy participants and patients with various diseases. Cochrane Database Syst. Rev. 2012, 3. [Google Scholar] [CrossRef]
- Moser, U. Vitamins—Wrong approaches. Int. J. Vitam. Nutr. Res. 2012, 82, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.A.; García-Bailo, B.; Badawi, A.; El-Sohemy, A. Genetic determinants of dietary antioxidant status. Prog. Mol. Biol. Transl. Sci. 2012, 108, 179–200. [Google Scholar] [PubMed]
- Dolara, P.; Bigagli, E.; Collins, A. Antioxidant vitamins and mineral supplementation, life span expansion and cancer incidence: A critical commentary. Eur. J. Nutr. 2012, 51, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Bjelakovic, G.; Nikolova, D.; Gluud, C. Meta-regression analyses, meta-analyses, and trial sequential analyses of the effects of supplementation with beta-carotene, vitamin A, and vitamin E singly or in different combinations on all-cause mortality: Do we have evidence for lack of harm? PLoS ONE 2013, 8, e74558. [Google Scholar] [CrossRef] [PubMed]
- Boeing, H.; Bechthold, A.; Bub, A.; Ellinger, S.; Haller, D.; Kroke, A.; Leschik-Bonnet, E.; Muller, M.J.; Oberritter, H.; Schulze, M.; et al. Critical review: Vegetables and fruit in the prevention of chronic diseases. Eur. J. Nutr. 2012, 51, 637–663. [Google Scholar] [CrossRef] [PubMed]
- Rink, S.M.; Mendola, P.; Mumford, S.L.; Poudrier, J.K.; Browne, R.W.; Wactawski-Wende, J.; Perkins, N.J.; Schisterman, E.F. Self-report of fruit and vegetable intake that meets the 5 a day recommendation is associated with reduced levels of oxidative stress biomarkers and increased levels of antioxidant defense in premenopausal women. J. Acad. Nutr. Diet. 2013, 113, 776–785. [Google Scholar] [CrossRef] [PubMed]
- Jenab, M.; Slimani, N.; Bictash, M.; Ferrari, P.; Bingham, S.A. Biomarkers in nutritional epidemiology: Applications, needs and new horizons. Hum. Genet. 2009, 125, 507–525. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.H.J.M.; Ujcic-Voortman, J.K.; Uitenbroek, D.G. Vitamin A and E status of the Population of Amsterdan (in Dutch); Report 5.05-06.4.1-4 to the Ministry of Public Health; Welfare and Sports: Bilthoven, The Netherlands, 2006. [Google Scholar]
- Tanumihardjo, S.A. Vitamin A: Biomarkers of nutrition for development. Am. J. Clin. Nutr. 2011, 94, 658S–665S. [Google Scholar] [CrossRef] [PubMed]
- Campbell, D.R.; Gross, M.D.; Martini, M.C.; Grandits, G.A.; Slavin, J.L.; Potter, J.D. Plasma carotenoids as biomarkers of vegetable and fruit intake. Cancer Epidemiol. Biomark. Prev. 1994, 3, 493–500. [Google Scholar]
- Ros, M.M.; Bueno-de-Mesquita, H.B.; Kampman, E.; Aben, K.K.; Büchner, F.L.; Jansen, E.H.; van Gils, C.H.; Egevad, L.; Overvad, K.; Tjønneland, A.; et al. Plasma carotenoids and vitamin C concentrations and risk of urothelial cell carcinoma in the European Prospective Investigation into Cancer and Nutrition. Am. J. Clin. Nutr. 2012, 96, 902–910. [Google Scholar] [CrossRef] [PubMed]
- Voutilainen, S.; Nurmi, T.; Mursu, J.; Rissanen, T.H. Carotenoids and cardiovascular health. Am. J. Clin. Nutr. 2006, 83, 1265–1271. [Google Scholar] [PubMed]
- Hung, R.J.; Zhang, Z.F.; Rao, J.Y.; Pantuck, A.; Reuter, V.E.; Heber, D.; Lu, Q.Y. Protective effects of plasma carotenoids on the risk of bladder cancer. J. Urol. 2006, 176, 1192–1197. [Google Scholar] [CrossRef] [PubMed]
- Helzlsouer, K.J.; Comstock, G.W.; Morris, J.S. Selenium, lycopene, α-tocopherol, β-carotene, retinol, and subsequent bladder cancer. Cancer Res. 1989, 49, 6144–6148. [Google Scholar] [PubMed]
- Carr, A.C.; Frei, B. Toward a new recommended dietary allowance for vitamin C based on antioxidant and health effects in humans. Am. J. Clin. Nutr. 1999, 69, 1086–1107. [Google Scholar] [PubMed]
- Jacob, R.A.; Sotoudeh, G. Vitamin C function and status in chronic disease. Nutr. Clin. Care 2002, 5, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Steghens, J.P.; van Kappel, A.L.; Riboli, E.; Collombel, C. Simultaneous measurement of seven carotenoids, retinol and α-tocopherol in serum by high-performance liquid chromatography. J. Chromatogr. B 1997, 694, 71–81. [Google Scholar] [CrossRef]
- Al-Delaimy, W.K.; Slimani, N.; Ferrari, P.; Key, T.; Spencer, E.; Johansson, I.; Johansson, G.; Mattisson, I.; Wirfalt, E.; Sieri, S.; et al. Plasma carotenoids as biomarkers of intake of fruits and vegetables: Ecological-level correlations in the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Clin. Nutr. 2005, 59, 1397–1408. [Google Scholar] [CrossRef] [PubMed]
- Karlsen, A.; Blomhoff, R.; Gundersen, T.E. High-throughput analysis of vitamin C in human plasma with the use of HPLC with monolithic column and UV-detection. J. Chromatogr. B 2005, 824, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.; Roberts, S.M.; Labbe, R.F. Ascorbic acid determination with an automated enzymatic procedure. Clin. Chem. 1997, 43, 154–157. [Google Scholar] [PubMed]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.H.; Ruskovska, T. Comparative analysis of serum (anti)oxidative status parameters in healthy persons. Int. J. Mol. Sci. 2013, 14, 6106–6115. [Google Scholar] [CrossRef] [PubMed]
- Deramaudt, T.B.; Dill, C.; Bonay, M. Regulation of oxidative stress by Nrf2 in the pathophysiology of infectious diseases. Med. Mal. Infect. 2013, 43, 100–107. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.H.J.M.; Beekhof, P.K.; Viezeliene, D.; Muzakova, V.; Skalicky, J. Long term stability of cancer biomarkers of oxidative stress, redox status, homocysteine, CRP and liver enzymes in human serum. Biomark. Med. 2015, 9, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Leufkens, A.M.; van Duijnhoven, F.J.B.; Woudt, S.H.S.; Siersema, P.D.; Jenab, M.; Jansen, E.H.J.M.; Pischon, T.; Tjønneland, A.; Olsen, A.; Overvad, K.; et al. Biomarkers of oxidative stress and risk of developing colorectal cancer: A cohort-nested case-control study in the EPIC study. Am. J. Epidemiol. 2012, 175, 653–663. [Google Scholar] [CrossRef] [PubMed]
- Jansen, E.H.J.M.; Beekhof, P.K.; Cremers, J.W.J.M.; Viezeliene, D.; Muzakova, V.; Skalicky, J. Short-term stability of biomarkers of oxidative stress and antioxidant status in human serum. ISRN Biomark. 2013, 2013. [Google Scholar] [CrossRef]
- Ruskovska, T.; Jansen, E.; Velickova, N.; Kamcev, N.; Beekhof, P.; Rodenburg, W. Circadian rhythm of (anti)oxidant biomarkers in human serum. PLoS ONE 2016. Manuscript in preparation. [Google Scholar]
- Boffetta, P.; Bobak, M.; Borsch-Supan, A.; Brenner, H.; Eriksson, S.; Grodstein, F.; Jansen, E.; Jenab, M.; Juerges, H.; Kampman, E.; et al. The Consortium on Health and Ageing: Network of Cohorts in Europe and the United States (CHANCES) project. Design, population and data harmonization of a large-scale, international study. Eur. J. Epidemiol. 2014, 29, 929–936. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Bianchi, S.; Battaglia, D.; Landi, P.; Bianchi, F.; Carpeggiani, C. Elevated levels of oxidative stress as a prognostic predictor of major adverse cardiovascular events in patients with coronary artery disease. J. Atheroscler. Thromb. 2012, 19, 712–717. [Google Scholar] [CrossRef] [PubMed]
- Vassalle, C.; Bianchi, S.; Bianchi, F.; Landi, P.; Battaglia, D.; Carpeggiani, C. Oxidative stress as a predictor of cardiovascular events in coronary artery disease patients. Clin. Chem. Lab. Med. 2012, 50, 1463–1468. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Drogan, D.; Boeing, H.; Jenab, M.; Bueno-de-Mesquita, H.B.; Jansen, E.; van Duijnhoven, F.J.B.; Rinaldi, S.; Fedirko, V.; Romieu, I.; et al. Adiposity, mediating biomarkers and risk of colon cancer in the European prospective investigation into cancer and nutrition study. Int. J. Cancer 2014, 134, 612–621. [Google Scholar] [CrossRef] [PubMed]
- Aleksandrova, K.; Jenab, M.; Bueno-de-Mesquita, H.B.; Fedirko, V.; Kaaks, R.; Lukanova, A.; van Duijnhoven, F.J.B.; Jansen, E.; Rinaldi, S.; Romieu, I.; et al. Biomarker patterns of inflammatory and metabolic pathways are associated with risk of colorectal cancer: Results from the European Prospective Investigation into Cancer and Nutrition (EPIC). Eur. J. Epidemiol. 2014, 29, 261–275. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Zhang, Y.; Heiss, J.A.; Butterbach, K.; Jansen, E.H.J.M.; Bewerunge-Hudler, M.; Saum, H.-U.; Holleczek, B.; Brenner, H. Discovery of a novel epigenetic cancer marker related to the oxidative status of human blood. Genes Chromosom. Cancer 2015, 54, 583–594. [Google Scholar] [CrossRef] [PubMed]
- Schöttker, B.; Saum, K.U.; Jansen, E.H.J.M.; Boffetta, P.; Trichopoulou, A.; Holleczeck, B.; Brenner, H. Oxidative stress markers and all-cause mortality at older age: A population-based cohort study. J. Gerontol. A Biol. Sci. Med. Sci. 2015, 70, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Saum, K.U.; Dieffenbach, A.K.; Jansen, E.H.J.M.; Holleczek, B.; Hauer, K.; Brenner, H. Association between oxidative stress and frailty in an elderly German population: Results from the ESTHER cohort study. Gerontology 2015, 61, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, C.M.; Dos Santos, R.C.C.; Lima, E.S. A simple automated procedure for thiol measurement in human serum samples. J. Bras. Patol. Med. Lab. 2006, 42, 345–350. [Google Scholar] [CrossRef]
- Ellman, G.L. Tissue sulfhydryl groups. Arch. Biochem. Biophys. 1959, 82, 70–77. [Google Scholar] [CrossRef]
- Hu, M.L. Measurement of protein thiol groups and glutathione in plasma. Methods Enzymol. 1994, 233, 380–385. [Google Scholar] [PubMed]
- Hedrick, V.E.; Dietrich, A.M.; Estabrooks, P.A.; Savla, J.; Serrano, E.; Davy, B.M. Dietary biomarkers: Advances, limitations and future directions. Nutr. J. 2012, 11. [Google Scholar] [CrossRef] [PubMed]
- Combs, G.F.; Trumbo, P.R.; McKinley, M.C.; Milner, J.; Studenski, S.; Kimura, T.; Watkins, S.M.; Raiten, D.J. Biomarkers in nutrition: New frontiers in research and application. Ann. N. Y. Acad. Sci. 2013, 1278, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Nanrib, H.; Nakamurab, K.; Harab, M.; Higakib, Y.; Imaizumib, T.; Taguchi, N.; Sakamoto, T.; Horita, M.; Shinchi, K.; Tanaka, K. Association between dietary pattern and serum C-reactive protein in Japanese men and women. J. Epidemiol. 2011, 21, 122–131. [Google Scholar] [CrossRef]
- Lettieri-Barbato, D.; Tomei, F.; Sancini, A.; Morabito, G.; Serafini, M. Effect of plant foods and beverages on plasma non-enzymatic antioxidant capacity in human subjects: A meta-analysis. Br. J. Nutr. 2013, 109, 1544–1556. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.K.; Cho, S.W.; Park, Y.K. Long-term vegetarians have low oxidative stress, body fat, and cholesterol levels. Nutr. Res. Pract. 2012, 6, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Yuan, L.; Meng, L.; Ma, W.; Xiao, Z.; Zhu, X.; Feng, J.F.; Yu, H.L.; Xiao, R. Impact of apple and grape juice consumption on the antioxidant status in healthy subjects. Int. J. Food Sci. Nutr. 2011, 62, 844–850. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, M.; Lee, S.G.; Davis, C.G.; Koo, S.I.; Chun, O.K. Dietary total antioxidant capacity is associated with diet and plasma antioxidant status in healthy young adults. J. Acad. Nutr. Diet. 2012, 112, 1626–1635. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.B. Vitamins, trace minerals, and other micronutrients. In Cecil Medicine, 23rd; Goldman, L., Ausiello, D., Eds.; Saunders Elsevier: Philadelphia, PA, USA, 2007. [Google Scholar]
- Burtis, C.A.; Ashwood, E.R.; Burns, D.E. Tietz Textbook of Clinical Chemistry and Molecular Diagnostics, 4th ed.; Elsevier Saunders: St. Louis, MO, USA, 2006. [Google Scholar]
- Wright, M.E.; Lawson, K.A.; Weinstein, S.J.; Pietinen, P.; Taylor, P.R.; Virtamo, J.; Albanes, D. Higher baseline serum concentrations of vitamin E are associated with lower total and cause-specific mortality in the α-Tocopherol, β-Carotene Cancer Prevention Study. Am. J. Clin. Nutr. 2006, 84, 1200–1207. [Google Scholar] [PubMed]
- Olmedilla, B.; Granado, F.; Gil-Martinez, E.; Blanco, I.; Rojas-Hidalgo, E. Reference values for retinol, tocopherol, and main carotenoids in serum of control and insulin dependent diabetic Spanish subjects. Clin. Chem. 1997, 43, 1066–1071. [Google Scholar] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jansen, E.; Ruskovska, T. Serum Biomarkers of (Anti)Oxidant Status for Epidemiological Studies. Int. J. Mol. Sci. 2015, 16, 27378-27390. https://doi.org/10.3390/ijms161126032
Jansen E, Ruskovska T. Serum Biomarkers of (Anti)Oxidant Status for Epidemiological Studies. International Journal of Molecular Sciences. 2015; 16(11):27378-27390. https://doi.org/10.3390/ijms161126032
Chicago/Turabian StyleJansen, Eugène, and Tatjana Ruskovska. 2015. "Serum Biomarkers of (Anti)Oxidant Status for Epidemiological Studies" International Journal of Molecular Sciences 16, no. 11: 27378-27390. https://doi.org/10.3390/ijms161126032
APA StyleJansen, E., & Ruskovska, T. (2015). Serum Biomarkers of (Anti)Oxidant Status for Epidemiological Studies. International Journal of Molecular Sciences, 16(11), 27378-27390. https://doi.org/10.3390/ijms161126032