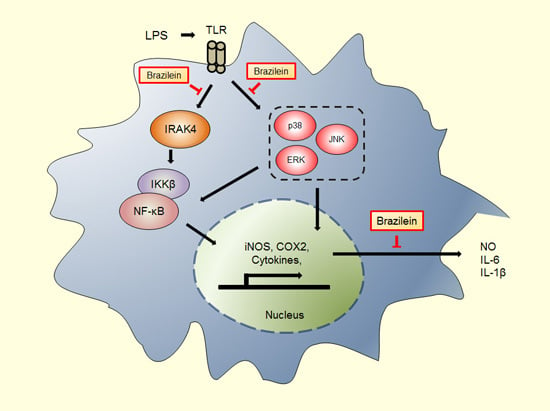
Brazilein Suppresses Inflammation through Inactivation of IRAK4-NF-κB Pathway in LPS-Induced Raw264.7 Macrophage Cells
Abstract
:1. Introduction
2. Results
2.1. Effect of Brazilein on the Inflammatory Products in LPS-Induced Raw264.7

2.2. Brazilein Attenuates the mRNA Levels of Pro-Inflammatory Cytokines in LPS-Induced Raw264.7


2.3. Brazilein Decreased NF-κB Luciferase Activity in LPS-Induced Raw264.7

2.4. Brazilein Inhibited the Upstream Target of NF-κB in LPS-Stimulated Raw264.7

3. Discussion
4. Experimental Section
4.1. Materials
4.2. Cell Line
4.3. Evaluation of the Cell Cytotoxicity
4.4. Transient Transfection and Luciferase Assays
4.5. Nitrite Colorimetric Assay
4.6. RNA Extraction and Semi-Quantitative RT-PCR
4.7. Enzyme-Linked Immunosorbent Assay (ELISA)
4.8. Western Blotting
4.9. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Villiger, P.M.; Terkeltaub, R.; Lotz, M. Production of monocyte chemoattractant protein-1 by inflamed synovial tissue and cultured synoviocytes. J. Immunol. 1992, 149, 722–727. [Google Scholar] [PubMed]
- Malyshev, I.Y.; Shnyra, A. Controlled modulation of inflammatory, stress and apoptotic responses in macrophages. Curr. Drug Targets Immune Endocr. Metab. Disord. 2003, 3, 1–22. [Google Scholar] [CrossRef]
- Lynch, M.A. The multifaceted profile of activated microglia. Mol. Neurobiol. 2009, 40, 139–156. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.L.; Cosseau, C.; Gardy, J.L.; Hancock, R.E. Complexities of targeting innate immunity to treat infection. Trends Immunol. 2007, 28, 260–266. [Google Scholar] [CrossRef] [PubMed]
- O'Neill, L.A. Tlrs: Professor mechnikov, sit on your hat. Trends Immunol. 2004, 25, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Beutler, B.; Rietschel, E.T. Innate immune sensing and its roots: The story of endotoxin. Nat. Rev. Immunol. 2003, 3, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.C.; Yeh, W.C.; Ohashi, P.S. Lps/tlr4 signal transduction pathway. Cytokine 2008, 42, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Takeda, K. Toll-like receptor signalling. Nat. Rev. Immunol. 2004, 4, 499–511. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Akira, S. Tlr signaling. Cell Death Differ. 2006, 13, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Gohda, J.; Matsumura, T.; Inoue, J. Cutting edge: TNFR-associated factor (TRAF) 6 is essential for MyD88-dependent pathway but not Toll/il-1 receptor domain-containing adaptor-inducing IFN-β (TRIF)-dependent pathway in TLR signaling. J. Immunol. 2004, 173, 2913–2917. [Google Scholar] [CrossRef] [PubMed]
- Sato, S.; Sanjo, H.; Takeda, K.; Ninomiya-Tsuji, J.; Yamamoto, M.; Kawai, T.; Matsumoto, K.; Takeuchi, O.; Akira, S. Essential function for the kinase TAK1 in innate and adaptive immune responses. Nat. Immunol. 2005, 6, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Keating, S.E.; Maloney, G.M.; Moran, E.M.; Bowie, A.G. IRAK-2 participates in multiple Toll-like receptor signaling pathways to NFκB via activation of TRAF6 ubiquitination. J. Biol. Chem. 2007, 282, 33435–33443. [Google Scholar] [CrossRef] [PubMed]
- Takaesu, G.; Ninomiya-Tsuji, J.; Kishida, S.; Li, X.; Stark, G.R.; Matsumoto, K. Interleukin-1 (IL-1) receptor-associated kinase leads to activation of TAK1 by inducing TAB2 translocation in the IL-1 signaling pathway. Mol. Cell. Biol. 2001, 21, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Karin, M. Mammalian map kinase signalling cascades. Nature 2001, 410, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.M.; Maniatis, T. Ikkepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Suemori, H.; Hata, N.; Asagiri, M.; Ogasawara, K.; Nakao, K.; Nakaya, T.; Katsuki, M.; Noguchi, S.; Tanaka, N.; et al. Distinct and essential roles of transcription factors IRF-3 and IRF-7 in response to viruses for IFN-α/β gene induction. Immunity 2000, 13, 539–548. [Google Scholar] [CrossRef]
- Wang, X.; Sakuma, T.; Asafu-Adjaye, E.; Shiu, G.K. Determination of ginsenosides in plant extracts from panax ginseng and panax quinquefolius l. By lc/ms/ms. Anal. Chem. 1999, 71, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Goh, F.G.; Midwood, K.S. Intrinsic danger: Activation of Toll-like receptors in rheumatoid arthritis. Rheumatology 2012, 51, 7–23. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Soto, J.C.; Garcia-Vazquez, F.A.; Gumbao, D.; Landeras, J.; Gadea, J. Assessment of two thawing processes of cryopreserved human sperm in pellets. Cryobiology 2012, 63, 131–136. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, Y.; Hosokawa, T.; Nagai, M.; Nagumo, S. In vitro study for inhibition of no production about constituents of sappan lignum. Biol. Pharm. Bull. 2007, 30, 193–196. [Google Scholar] [CrossRef] [PubMed]
- Bae, I.K.; Min, H.Y.; Han, A.R.; Seo, E.K.; Lee, S.K. Suppression of lipopolysaccharide-induced expression of inducible nitric oxide synthase by brazilin in raw 264.7 macrophage cells. Eur. J. Pharmacol. 2005, 513, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Shen, J.; Zhang, H.; Lin, H.; Su, H.; Xing, D.; Du, L. Brazilein protects the brain against focal cerebral ischemia reperfusion injury correlating to inflammatory response suppression. Eur. J. Pharmacol. 2007, 558, 88–95. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Xie, W.D.; Lei, F.; Meng, Z.; Zhao, Y.N.; Su, H.; Du, L.J. Brazilein, an important immunosuppressive component from caesalpinia sappan l. Int. Immunopharmacol. 2006, 6, 426–432. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Wu, B.; Pan, Y.J.; Zheng, S. Brazilein inhibits survivin protein and mRNA expression and induces apoptosis in hepatocellular carcinoma HepG2 cells. Neoplasma 2009, 56, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Tak, P.P.; Firestein, G.S. Nf-kappab: A key role in inflammatory diseases. J. Clin. Investig. 2001, 107, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Oz, H.S.; Barve, S.; de Villiers, W.J.; McClain, C.J.; Varilek, G.W. The green tea polyphenol (-)-epigallocatechin-3-gallate blocks nuclear factor-βB activation by inhibiting I βB kinase activity in the intestinal epithelial cell line IEC-6. Mol. Pharmacol. 2001, 60, 528–533. [Google Scholar] [PubMed]
- Pan, M.H.; Lin-Shiau, S.Y.; Ho, C.T.; Lin, J.H.; Lin, J.K. Suppression of lipopolysaccharide-induced nuclear factor-kappab activity by theaflavin-3,3'-digallate from black tea and other polyphenols through down-regulation of ikappab kinase activity in macrophages. Biochem. Pharmacol. 2000, 59, 357–367. [Google Scholar] [CrossRef]
- Hsieh, C.Y.; Tsai, P.C.; Chu, C.L.; Chang, F.R.; Chang, L.S.; Wu, Y.C.; Lin, S.R. Brazilein suppresses migration and invasion of MDA-MB-231 breast cancer cells. Chem. Biol. Interact. 2013, 204, 105–115. [Google Scholar] [CrossRef] [PubMed]
- Schulze-Osthoff, K.; Ferrari, D.; Riehemann, K.; Wesselborg, S. Regulation of NF-βB activation by map kinase cascades. Immunobiology 1997, 198, 35–49. [Google Scholar] [CrossRef]
- Fujioka, S.; Niu, J.; Schmidt, C.; Sclabas, G.M.; Peng, B.; Uwagawa, T.; Li, Z.; Evans, D.B.; Abbruzzese, J.L.; Chiao, P.J. NF-βB and AP-1 connection: Mechanism of NF-βB-dependent regulation of AP-1 activity. Mol. Cell. Biol. 2004, 24, 7806–7819. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.L.; Xiao, S.; Guo, Y. Activation of transcription factors NF-βB and AP-1 and their relations with apoptosis associated-proteins in hepatocellular carcinoma. World J. Gastroenterol. 2005, 11, 3860–3865. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.J.; Choi, H.S.; Yoon, K.Y.; Lee, O.H.; Kim, K.J.; Lee, B.Y. Oleuropein suppresses lps-induced inflammatory responses in raw 264.7 cell and zebrafish. J. Agric. Food Chem. 2015, 63, 2098–2105. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.J.; Yoon, K.Y.; Lee, B.Y. Low molecular weight fucoidan from the sporophyll of undaria pinnatifida suppresses inflammation by promoting the inhibition of mitogen-activated protein kinases and oxidative stress in raw264.7 cells. Fitoterapia 2012, 83, 1628–1635. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.J.; Huang, S.S.; Deng, J.S. Anti-inflammatory activities of inotilone from phellinus linteus through the inhibition of MMP-9, NF-βB, and MAPK activation in vitro and in vivo. PLoS ONE 2012, 7, e35922. [Google Scholar] [CrossRef] [PubMed]
- Shao, H.J.; Jeong, J.B.; Kim, K.J.; Lee, S.H. Anti-inflammatory activity of mushroom-derived hispidin through blocking of nf-kappab activation. J. Sci. Food Agric. 2015, 95, 2482–2486. [Google Scholar] [CrossRef] [PubMed]
- Li, X. IRAK4 in TLR/IL-1R signaling: Possible clinical applications. Eur. J. Immunol. 2008, 38, 614–618. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.R.; Kim, D.S.; Lee, I.S.; Jung, K.Y.; Lee, J.J.; Lee, H.K. Anticomplementary activity of constituents from the heartwood of caesalpinia sappan. Planta Med. 1998, 64, 456–458. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, K.-J.; Yoon, K.-Y.; Yoon, H.-S.; Oh, S.-R.; Lee, B.-Y. Brazilein Suppresses Inflammation through Inactivation of IRAK4-NF-κB Pathway in LPS-Induced Raw264.7 Macrophage Cells. Int. J. Mol. Sci. 2015, 16, 27589-27598. https://doi.org/10.3390/ijms161126048
Kim K-J, Yoon K-Y, Yoon H-S, Oh S-R, Lee B-Y. Brazilein Suppresses Inflammation through Inactivation of IRAK4-NF-κB Pathway in LPS-Induced Raw264.7 Macrophage Cells. International Journal of Molecular Sciences. 2015; 16(11):27589-27598. https://doi.org/10.3390/ijms161126048
Chicago/Turabian StyleKim, Kui-Jin, Kye-Yoon Yoon, Hyung-Sun Yoon, Sei-Ryang Oh, and Boo-Yong Lee. 2015. "Brazilein Suppresses Inflammation through Inactivation of IRAK4-NF-κB Pathway in LPS-Induced Raw264.7 Macrophage Cells" International Journal of Molecular Sciences 16, no. 11: 27589-27598. https://doi.org/10.3390/ijms161126048