A Variant in the Osteoprotegerin Gene Is Associated with Coronary Atherosclerosis in Patients with Rheumatoid Arthritis: Results from a Candidate Gene Study
Abstract
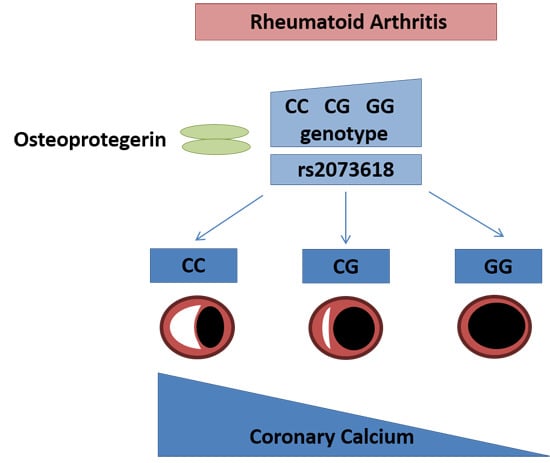
:1. Introduction
2. Results
| Patient Characteristics | With Coronary Calcium (n = 70) | Without Coronary Calcium (n = 70) | p-Value |
|---|---|---|---|
| Age (years) | 60 ± 9 | 48 ± 10 | <0.001 |
| Female sex (%) | 59% | 80% | 0.01 |
| Caucasian | 90% | 87% | 0.40 |
| Disease duration (years) | 11 ± 11 | 6 ± 8 | 0.03 |
| Systolic blood pressure | 138 ± 21 | 129 ± 17 | 0.02 |
| Diastolic blood pressure | 76 ± 11 | 75 ± 10 | 0.24 |
| Body mass index | 29 ± 6 | 30 ± 7 | 0.64 |
| Total cholesterol | 184 ± 38 | 184 ± 41 | 0.67 |
| LDL cholesterol | 113 ± 27 | 109 ± 32 | 0.42 |
| SNP | Gene | Major, Minor Allele | Minor Allele Frequency | Odds Ratio * (95% C.I.) | p-Value |
|---|---|---|---|---|---|
| rs2073618 ** | TNFRSF11B | G,C | 0.36 | 4.09 (1.93–8.70) | 0.00026 |
| rs522616 | MMP3 | T,C | 0.29 | 4.43 (1.77–11.10) | 0.001 |
| rs2853694 | IL12B | T,C | 0.29 | 3.09 (1.53–6.24) | 0.002 |
| rs3918249 | MMP9 | T,C | 0.49 | 0.36 (0.18–0.69) | 0.002 |
| rs17576 | MMP9 | A,G | 0.45 | 0.34 (0.17–0.68) | 0.002 |
| rs3918253 | MMP9 | C,T | 0.28 | 0.38 (0.20–0.72) | 0.003 |
| rs2274756 | MMP9 | G,A | 0.16 | 0.24 (0.09–0.64) | 0.004 |
| rs751271 | NOD2 | T,G | 0.49 | 2.57 (1.33–4.96) | 0.005 |
| rs650108 | MMP3 | G,A | 0.41 | 2.82 (1.29–6.17) | 0.009 |
| rs1800947 | CRP | C,G | 0.04 | 5.93 (1.43–24.71) | 0.014 |
| rs9562414 | TNFSF11 | A,G | 0.06 | 0.25 (0.08–0.79) | 0.019 |
| rs2107545 | MPO | T,C | 0.26 | 0.42 (0.20–0.89) | 0.023 |
| rs3745367 | RETN | G,A | 0.39 | 0.45 (0.22–0.91) | 0.026 |
| rs10954213 | IRF5 | G,A | 0.47 | 0.50 (0.27–0.94) | 0.030 |
| rs633137 | TNFSF11 | T,A | 0.08 | 0.34 (0.13–0.91) | 0.031 |
| rs2243828 | MPO | A,G | 0.23 | 0.43 (0.20–0.96) | 0.040 |
| rs1801274 | FCGR2A | A,G | 0.43 | 0.52 (0.28–1.00) | 0.049 |

3. Discussion
4. Methods
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Solomon, D.H.; Karlson, E.W.; Rimm, E.B.; Cannuscio, C.C.; Mandl, L.A.; Manson, J.E.; Stampfer, M.J.; Curhan, G.C. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation 2003, 107, 1303–1307. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Oeser, A.; Avalos, I.; Gebretsadik, T.; Shintani, A.; Raggi, P.; Sokka, T.; Pincus, T.; Stein, C.M. Utility of the Framingham risk score to predict the presence of coronary atherosclerosis in patients with rheumatoid arthritis. Arthritis Res. Ther. 2006, 8, R186. [Google Scholar] [CrossRef] [PubMed]
- Chung, C.P.; Oeser, A.; Raggi, P.; Gebretsadik, T.; Shintani, A.K.; Sokka, T. Increased coronary-artery atherosclerosis in rheumatoid arthritis: Relationship to disease duration and cardiovascular risk factors. Arthritis Rheumatol. 2005, 52, 3045–3053. [Google Scholar] [CrossRef]
- Del Rincon, I.D.; Willliams, K.; Stern, M.P.; Freeman, G.L.; Escalante, A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheumatol. 2001, 44, 2737–2745. [Google Scholar] [CrossRef]
- Gonzalez-Gay, M.A.; Gonzalez-Juanatey, C.; Pineiro, A.; Garcia-Porrua, C.; Testa, A.; Llorca, J. High-grade C-reactive protein elevation correlates with accelerated atherogenesis in patients with rheumatoid arthritis. J. Rheumatol. 2005, 32, 1219–1223. [Google Scholar] [PubMed]
- Rodriguez-Rodriguez, L.; Lopez-Mejias, R.; Garcia-Bermudez, M.; Gonzalez-Juanatey, C.; Gonzalez-Gay, M.A.; Martin, J. Genetic markers of cardiovascular disease in rheumatoid arthritis. Mediat. Inflamm. 2012, 2012, 574817. [Google Scholar]
- Palomino-Morales, R.; Gonzalez-Juanatey, C.; Vazquez-Rodriguez, T.R.; Rodriguez, L.; Miranda-Filoy, J.A.; Llorca, J.; Martin, J.; Gonzalez-Gay, M.A. A1298C polymorphism in the MTHFR gene predisposes to cardiovascular risk in rheumatoid arthritis. Arthritis Res. Ther. 2010, 12, R71. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Rodriguez, L.; Gonzalez-Juanatey, C.; Palomino-Morales, R.; Vázquez-Rodríguez, T.R.; Miranda-Filloy, J.A.; Fernández-Gutiérrez, B.; Llorca, J.; Martin, J.; González-Gay, M.A. TNFA-308 (rs1800629) polymorphism is associated with a higher risk of cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 2011, 216, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Mejias, R.; Genre, F.; Garcia-Bermudez, M.; Corrales, A.; González-Juanatey, C.; Llorca, J.; Miranda-Filloy, J.A.; Rueda-Gotor, J.; Blanco, R.; Castañeda, S.; et al. The ZC3HC1 rs11556924 polymorphism is associated with increased carotid intima-media thickness in patients with rheumatoid arthritis. Arthritis Res. Ther. 2013, 15, R152. [Google Scholar]
- Lopez-Mejias, R.; Genre, F.; Garcia-Bermudez, M.; Castañeda, S.; González-Juanatey, C.; Llorca, J.; Corrales, A.; Miranda-Filloy, J.A.; Rueda-Gotor, J.; Gómez-Vaquero, C.; Rodríguez-Rodríguez, L.; et al. The 11q23.3 genomic region—rs964184—is associated with cardiovascular disease in patients with rheumatoid arthritis. Tissue Antigens 2013, 82, 344–347. [Google Scholar]
- Lopez-Mejias, R.; Garcia-Bermudez, M.; Gonzalez-Juanatey, C.; Castañeda, S.; Miranda-Filloy, J.A.; Gómez-Vaquero, C.; Fernández-Gutiérrez, B.; Balsa, A.; Pascual-Salcedo, D.; Blanco, R.; et al. NFKB1-94ATTG ins/del polymorphism (rs28362491) is associated with cardiovascular disease in patients with rheumatoid arthritis. Atherosclerosis 2012, 224, 426–429. [Google Scholar]
- Asanuma, Y.; Chung, C.P.; Oeser, A.; Solus, J.F.; Avalos, I.; Gebretsadik, T.; Shintani, A.; Raggi, P.; Sokka, T.; Pincus, T.; Stein, C.M. Serum osteoprotegerin is increased and independently associated with coronary-artery atherosclerosis in patients with rheumatoid arthritis. Atherosclerosis 2007, 195, e135–e141. [Google Scholar] [CrossRef] [PubMed]
- Simonet, W.S.; Lacey, D.L.; Dunstan, C.R.; Kelley, M.; Chang, M.S.; Luthy, R.; Nguyen, H.Q.; Wooden, S.; Bennett, L.; Boone, T.; et al. Osteoprotegerin: A novel secreted protein involved in the regulation of bone density. Cell 1997, 89, 309–319. [Google Scholar]
- Reich, D.E.; Cargill, M.; Bolk, S.; Ireland, J.; Sabeti, P.C.; Richter, D.J.; Lavery, T.; Kouyoumjian, R.; Farhadian, S.F.; Ward, R.; et al. Linkage disequilibrium in the human genome. Nature 2001, 411, 199–204. [Google Scholar]
- Jono, S.; Ikari, Y.; Shioi, A.; Mori, K.; Miki, T.; Hara, K.; Nishizawa, Y. Serum osteoprotegerin levels are associated with the presence and severity of coronary artery disease. Circulation 2002, 106, 1192–1194. [Google Scholar] [CrossRef] [PubMed]
- Ziolkowska, M.; Kurowska, M.; Radzikowska, A.; Luszczykiewicz, G.; Wiland, P.; Dziewczopolski, W.; Filipowicz-Sosnowska, A.; Pazdur, J.; Szechinski, J.; Kowalczewski, J.; et al. High levels of osteoprotegerin and soluble receptor activator of nuclear factor kappa B ligand in serum of rheumatoid arthritis patients and their normalization after anti-tumor necrosis factor alpha treatment. Arthritis Rheumatol. 2002, 46, 1744–1753. [Google Scholar]
- Genre, F.; Lopez-Mejias, R.; Miranda-Filloy, J.A.; Ubilla, B.; Carnero-Lopez, B.; Palmou-Fontana, N.; Gomez-Acebo, I.; Blanco, R.; Rueda-Gotor, J.; Pina, T.; et al. Osteoprotegerin correlates with disease activity and endothelial activation in non-diabetic ankylosing spondylitis patients undergoing TNF-α antagonist therapy. Clin. Exp. Rheumatol. 2014, 32, 640–646. [Google Scholar]
- Genre, F.; Lopez-Mejias, R.; Garcia-Bermudez, M.; Castaneda, S.; Gonzalez-Juanatey, C.; Llorca, J.; Corrales, A.; Ubilla, B.; Miranda-Filloy, J.A.; Pina, T.; et al. Osteoprotegerin CGA haplotype protection against cerebrovascular complications in anti-CCP negative patients with rheumatoid arthritis. PLoS One 2014, 9, e106823. [Google Scholar]
- Biscetti, F.; Straface, G.; Giovannini, S.; Santoliquido, A.; Angelini, F.; Santoro, L.; Porreca, C.F.; Pecorini, G.; Ghirlanda, G.; Flex, A.; et al. Association between TNFRSF11B gene polymorphisms and history of ischemic stroke in Italian diabetic patients. Hum. Genet. 2013, 132, 49–55. [Google Scholar]
- Straface, G.; Biscetti, F.; Pitocco, D.; Bertoletti, G.; Misuraca, M.; Vincenzoni, C.; Snider, F.; Arena, V.; Stigliano, E.; Angelini, F.; et al. Assessment of the genetic effects of polymorphisms in the osteoprotegerin gene, TNFRSF11B, on serum osteoprotegerin levels and carotid plaque vulnerability. Stroke 2011, 42, 3022–3028. [Google Scholar]
- Lopez-Mejias, R.; Ubilla, B.; Genre, F.; Corrales, A.; Hernandez, J.L.; Ferraz-Amaro, I.; Tsang, L.; Llorca, J.; Blanco, R.; Gonzalez-Juanatey, C.; et al. Osteoprotegerin concentrations relate independently to established cardiovascular disease in rheumatoid arthritis. J. Rheumatol. 2015, 42, 39–45. [Google Scholar]
- Dessein, P.H.; Lopez-Mejias, R.; Gonzalez-Juanatey, C.; Genre, F.; Miranda-Filloy, J.A.; Llorca, J.; Gonzalez-Gay, M.A. Independent relationship of osteoprotegerin concentrations with endothelial activation and carotid atherosclerosis in patients with severe rheumatoid arthritis. J. Rheumatol. 2014, 41, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Juanatey, C.; Testa, A.; Garcia-Castelo, A.; Garcia-Porrua, C.; Llorca, J.; Vidan, J.; Hajeer, A.H.; Ollier, W.E.; Mattey, D.L.; Gonzalez-Gay, M.A. HLA-DRB1 status affects endothelial function in treated patients with rheumatoid arthritis. Am. J. Med. 2003, 114, 647–652. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Gay, M.A.; Gonzalez-Juanatey, C.; Llorca, J.; Ollier, W.E.; Martin, J. Contribution of HLA-DRB1 shared epitope alleles and chronic inflammation to the increased incidence of cardiovascular disease in rheumatoid arthritis: Comment on the article by Farragher et al. Arthritis Rheumatol. 2008, 58, 2584–2585. [Google Scholar] [CrossRef]
- Farragher, T.M.; Goodson, N.J.; Naseem, H.; Silman, A.J.; Thomson, W.; Symmons, D.; Barton, A. Association of the HLA-DRB1 gene with premature death, particularly from cardiovascular disease, in patients with rheumatoid arthritis and inflammatory polyarthritis. Arthritis Rheumatol. 2008, 58, 359–369. [Google Scholar] [CrossRef]
- Lopez-Mejias, R.; Garcia-Bermudez, M.; Gonzalez-Juanatey, C.; Castaneda, S.; Perez-Esteban, S.; Miranda-Filloy, J.A.; Gomez-Vaquero, C.; Fernandez-Gutierrez, B.; Balsa, A.; Pascual-Salcedo, D.; et al. Lack of association between IL6 single nucleotide polymorphisms and cardiovascular disease in Spanish patients with rheumatoid arthritis. Atherosclerosis 2011, 219, 655–658. [Google Scholar]
- Garcia-Bermudez, M.; Lopez-Mejias, R.; Gonzalez-Juanatey, C.; Castaneda, S.; Miranda-Filloy, J.A.; Blanco, R.; Fernandez-Gutierrez, B.; Balsa, A.; Gonzalez-Alvaro, I.; Gomez-Vaquero, C.; et al. Lack of association between TLR4 rs4986790 polymorphism and risk of cardiovascular disease in patients with rheumatoid arthritis. DNA Cell Biol. 2012, 31, 1214–1220. [Google Scholar]
- Garcia-Bermudez, M.; Lopez-Mejias, R.; Gonzalez-Juanatey, C.; Corrales, A.; Castaneda, S.; Ortiz, A.M.; Miranda-Filloy, J.A.; Gomez-Vaquero, C.; Fernandez-Gutierrez, B.; Balsa, A.; et al. CARD8 rs2043211 (p.C10X) polymorphism is not associated with disease susceptibility or cardiovascular events in Spanish rheumatoid arthritis patients. DNA Cell Biol. 2013, 32, 28–33. [Google Scholar]
- Garcia-Bermudez, M.; Lopez-Mejias, R.; Gonzalez-Juanatey, C.; Corrales, A.; Robledo, G.; Castaneda, S.; Miranda-Filloy, J.A.; Blanco, R.; Fernandez-Gutierrez, B.; Balsa, A.; et al. Analysis of the interferon gamma (rs2430561, +874T/A) functional gene variant in relation to the presence of cardiovascular events in rheumatoid arthritis. PLoS One 2012, 7, e47166. [Google Scholar]
- O’Donnell, C.J.; Kavousi, M.; Smith, A.V.; Kardia, S.L.; Feitosa, M.F.; Hwang, S.J.; Sun, Y.V.; Province, M.A.; Aspelund, T.; Dehghan, A.; et al. Genome-wide association study for coronary artery calcification with follow-up in myocardial infarction. Circulation 2011, 124, 2855–2864. [Google Scholar]
- Ferguson, J.F.; Matthews, G.J.; Townsend, R.R.; Raj, D.S.; Kanetsky, P.A.; Budoff, M.; Fischer, M.J.; Rosas, S.E.; Kanthety, R.; Rahman, M.; et al. Candidate gene association study of coronary artery calcification in chronic kidney disease: Findings from the CRIC study (Chronic Renal Insufficiency Cohort). J. Am. Coll. Cardiol. 2013, 62, 789–798. [Google Scholar]
- Lopez-Mejias, R.; Genre, F.; Corrales, A.; Gonzalez-Juanatey, C.; Ubilla, B.; Llorca, J.; Miranda-Filloy, J.A.; Pina, T.; Blanco, R.; Castaneda, S.; et al. Investigation of a PON1 gene polymorphism (rs662 polymorphism) as predictor of subclinical atherosclerosis in patients with rheumatoid arthritis. Ann. Rheum. Dis. 2014, 73, 1749–1750. [Google Scholar]
- Arnett, F.C.; Edworthy, S.M.; Bloch, D.A.; Mc Shane, D.J.; Fries, J.F.; Cooper, N.S.; Healey, L.A.; Kaplan, S.R.; Liang, M.H.; Luthra, H.S. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheumatol. 1988, 31, 315–324. [Google Scholar] [CrossRef]
- Prevoo, M.L.; van’t Hof, M.A.; Kuper, H.H.; van Leeuwen, M.A.; van de Putte, L.B.; van Riel, P.L. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheumatol. 1995, 38, 44–48. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, C.P.; Solus, J.F.; Oeser, A.; Li, C.; Raggi, P.; Smith, J.R.; Stein, C.M. A Variant in the Osteoprotegerin Gene Is Associated with Coronary Atherosclerosis in Patients with Rheumatoid Arthritis: Results from a Candidate Gene Study. Int. J. Mol. Sci. 2015, 16, 3885-3894. https://doi.org/10.3390/ijms16023885
Chung CP, Solus JF, Oeser A, Li C, Raggi P, Smith JR, Stein CM. A Variant in the Osteoprotegerin Gene Is Associated with Coronary Atherosclerosis in Patients with Rheumatoid Arthritis: Results from a Candidate Gene Study. International Journal of Molecular Sciences. 2015; 16(2):3885-3894. https://doi.org/10.3390/ijms16023885
Chicago/Turabian StyleChung, Cecilia P., Joseph F. Solus, Annette Oeser, Chun Li, Paolo Raggi, Jeffrey R. Smith, and C. Michael Stein. 2015. "A Variant in the Osteoprotegerin Gene Is Associated with Coronary Atherosclerosis in Patients with Rheumatoid Arthritis: Results from a Candidate Gene Study" International Journal of Molecular Sciences 16, no. 2: 3885-3894. https://doi.org/10.3390/ijms16023885
APA StyleChung, C. P., Solus, J. F., Oeser, A., Li, C., Raggi, P., Smith, J. R., & Stein, C. M. (2015). A Variant in the Osteoprotegerin Gene Is Associated with Coronary Atherosclerosis in Patients with Rheumatoid Arthritis: Results from a Candidate Gene Study. International Journal of Molecular Sciences, 16(2), 3885-3894. https://doi.org/10.3390/ijms16023885