Anti-Inflammatory Effect of Streptochlorin via TRIF-Dependent Signaling Pathways in Cellular and Mouse Models
Abstract
:1. Introduction
2. Results and Discussion
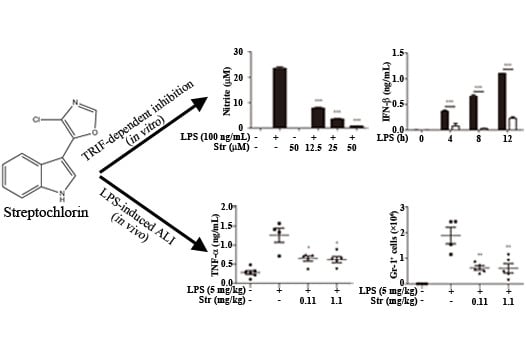
2.1. Streptochlorin Inhibited the Production of Proinflammatory Mediators in Lipopolysaccharide (LPS)-Stimulated RAW264.7 Cells


2.2. Streptochlorin Ameliorated LPS-Induced ALI in Mice

3. Experimental Section
3.1. Reagents and Antibodies
3.2. Cell Culture
3.3. Animals and LPS-Induced ALI Mouse Model
3.4. Real-Time Polymerase Chain Reaction (PCR)
3.5. Hematoxylin and Eosin (H&E) Staining
3.6. Other Experiments
3.7. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Huang, G.J.; Pan, C.H.; Liu, F.C.; Wu, T.S.; Wu, C.H. Anti-inflammatory effects of ethanolic extract of Antrodia salmonea in the lipopolysaccharide-stimulated RAW246.7 macrophages and the λ-carrageenan-induced paw edema model. Food Chem. Toxicol. 2012, 50, 1485–1493. [Google Scholar]
- Koppula, S.; Kim, W.; Jiang, J.; Shim, D.; Oh, N.; Kim, T.; Kang, T.; Lee, K. Carpesium macrocephalum attenuates lipopolysaccharide-induced inflammation in macrophages by regulating the NF-κB/IκB-α, Akt, and STAT signaling pathways. Am. J. Chin. Med. 2013, 41, 927–943. [Google Scholar] [CrossRef] [PubMed]
- Akira, S.; Uematsu, S.; Takeuchi, O. Pathogen recognition and innate immunity. Cell 2006, 124, 783–801. [Google Scholar] [CrossRef] [PubMed]
- Covert, M.W.; Leung, T.H.; Gaston, J.E.; Baltimore, D. Achieving stability of lipopolysaccharide-induced NF-κB activation. Science 2005, 309, 1854–1857. [Google Scholar] [CrossRef] [PubMed]
- Fitzgerald, K.A.; McWhirter, S.M.; Faia, K.L.; Rowe, D.C.; Latz, E.; Golenbock, D.T.; Coyle, A.J.; Liao, S.; Maniatis, T. IKKepsilon and TBK1 are essential components of the IRF3 signaling pathway. Nat. Immunol. 2003, 4, 491–496. [Google Scholar] [CrossRef] [PubMed]
- Bowie, A.G.; Haga, I.R. The role of Toll-like receptors in the host response to viruses. Mol. Immunol. 2005, 42, 859–867. [Google Scholar] [CrossRef] [PubMed]
- Perry, A.K.; Gang, C.; Zheng, D.; Hong, T.; Cheng, G. The host Type I interferon response to viral and bacterial infections. Cell Res. 2005, 15, 407–422. [Google Scholar] [CrossRef] [PubMed]
- Grommes, J.; Vijayan, S.; Drechsler, M.; Hartwig, H.; Mörgelin, M.; Dembinski, R.; Jacobs, M.; Koeppel, T.A.; Binnebösel, M.; Weber, C. Simvastatin reduces endotoxin-induced acute lung injury by decreasing neutrophil recruitment and radical formation. PLoS ONE 2012, 7, e38917. [Google Scholar] [CrossRef] [PubMed]
- Matute-Bello, G.; Frevert, C.W.; Martin, T.R. Animal models of acute lung injury. Am. J. Physiol. 2008, 295, L379–L399. [Google Scholar]
- Choi, I.K.; Shin, H.J.; Lee, H.S.; Kwon, H.J. Streptochlorin, a marine natural product, inhibits NF-κB activation and suppresses angiogenesis in vitro. J. Microbiol. Biotechnol. 2007, 17, 1338–1343. [Google Scholar] [PubMed]
- Shin, H.J.; Jeong, H.S.; Lee, H.S.; Park, S.K.; Kim, H.M.; Kwon, H.J. Isolation and structure determination of streptochlorin, an antiproliferative agent from a marine-derived Streptomyces sp. 04DH110. J. Microbiol. Biotechnol. 2007, 17, 1403–1406. [Google Scholar]
- Lee, S.; Shin, H.J.; Kim, D.; Shim, D.; Kim, T.; Ye, S.; Won, H.; Koppula, S.; Kang, T.; Lee, K. Streptochlorin suppresses allergic dermatitis and mast cell activation via regulation of Lyn/Fyn and Syk signaling pathways in cellular and mouse models. PLoS ONE 2013, 8, e74194. [Google Scholar] [CrossRef] [PubMed]
- Janeway, C.A., Jr.; Medzhitov, R. Innate immune recognition. Annu. Rev. Immunol. 2002, 20, 197–216. [Google Scholar] [CrossRef] [PubMed]
- Kawai, T.; Takeuchi, O.; Fujita, T.; Inoue, J.; Muhlradt, P.F.; Sato, S.; Hoshino, K.; Akira, S. Lipopolysaccharide stimulates the MyD88-independent pathway and results in activation of IFN-regulatory factor 3 and the expression of a subset of lipopolysaccharide-inducible genes. J. Immunol. 2001, 167, 5887–5894. [Google Scholar] [CrossRef] [PubMed]
- Toshchakov, V.; Jones, B.W.; Perera, P.; Thomas, K.; Cody, M.J.; Zhang, S.; Williams, B.R.; Major, J.; Hamilton, T.A.; Fenton, M.J. TLR4, but not TLR2, mediates IFN-Β-induced STAT1α/Β-dependent gene expression in macrophages. Nat. Immunol. 2002, 3, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Samavati, L.; Rastogi, R.; Du, W.; Hüttemann, M.; Fite, A.; Franchi, L. STAT3 tyrosine phosphorylation is critical for interleukin 1β and interleukin-6 production in response to lipopolysaccharide and live bacteria. Mol. Immunol. 2009, 46, 1867–1877. [Google Scholar] [CrossRef] [PubMed]
- Bode, J.G.; Ehlting, C.; Häussinger, D. The macrophage response towards LPS and its control through the p38MAPK–STAT3 axis. Cell Signal. 2012, 24, 1185–1194. [Google Scholar] [CrossRef] [PubMed]
- Knapp, S. LPS and bacterial lung inflammation models. Drug Discov. Today 2010, 6, 113–118. [Google Scholar]
- Adachi, O.; Kawai, T.; Takeda, K.; Matsumoto, M.; Tsutsui, H.; Sakagami, M.; Nakanishi, K.; Akira, S. Targeted disruption of the MyD88 gene results in loss of IL-1- and IL-18-mediated function. Immunity 1998, 9, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Shim, D.; Han, J.; Sun, X.; Jang, C.; Koppula, S.; Kim, T.; Kang, T.; Lee, K. Lysimachia clethroides Duby extract attenuates inflammatory response in RAW264.7 macrophages stimulated with lipopolysaccharide and in acute lung injury mouse model. J. Ethnopharmacol. 2013, 150, 1007–1015. [Google Scholar]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, D.-W.; Shin, H.J.; Han, J.-W.; Shin, W.-Y.; Sun, X.; Shim, E.-J.; Kim, T.-J.; Kang, T.-B.; Lee, K.-H. Anti-Inflammatory Effect of Streptochlorin via TRIF-Dependent Signaling Pathways in Cellular and Mouse Models. Int. J. Mol. Sci. 2015, 16, 6902-6910. https://doi.org/10.3390/ijms16046902
Shim D-W, Shin HJ, Han J-W, Shin W-Y, Sun X, Shim E-J, Kim T-J, Kang T-B, Lee K-H. Anti-Inflammatory Effect of Streptochlorin via TRIF-Dependent Signaling Pathways in Cellular and Mouse Models. International Journal of Molecular Sciences. 2015; 16(4):6902-6910. https://doi.org/10.3390/ijms16046902
Chicago/Turabian StyleShim, Do-Wan, Hee Jae Shin, Ji-Won Han, Woo-Young Shin, Xiao Sun, Eun-Jeong Shim, Tack-Joong Kim, Tae-Bong Kang, and Kwang-Ho Lee. 2015. "Anti-Inflammatory Effect of Streptochlorin via TRIF-Dependent Signaling Pathways in Cellular and Mouse Models" International Journal of Molecular Sciences 16, no. 4: 6902-6910. https://doi.org/10.3390/ijms16046902