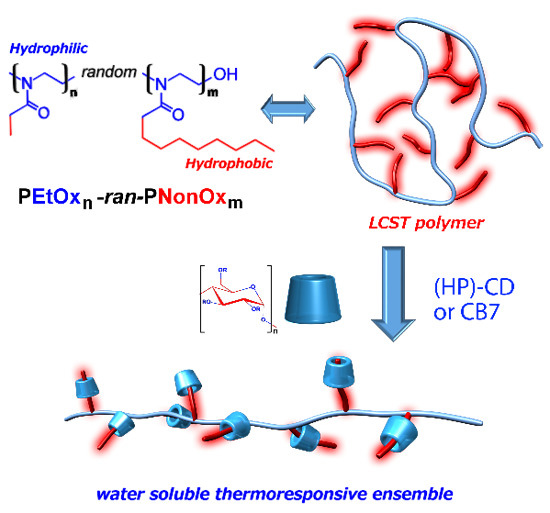
Thermoresponsive Interplay of Water Insoluble Poly(2-alkyl-2-oxazoline)s Composition and Supramolecular Host–Guest Interactions
Abstract
:1. Introduction

2. Results and Discussion
2.1. Poly(2-ethyl-2-oxazoline)-ran-poly(2-nonyl-2-oxazoline) Synthesis


| ID | Polymer Composition | DP | % NonOx (Number) | SEC | |
|---|---|---|---|---|---|
| Mn | Đ | ||||
| SN12 | PEtOx90-ran-PNonOx12 | 102 | 12 | 18,000 | 1.08 |
| SN20 | PEtOx84-ran-PNonOx21 | 105 | 20 | 17,500 | 1.08 |
| SN25 | PEtOx75-ran-PNonOx25 | 100 | 25 | 16,500 | 1.07 |
| SN33 | PEtOx62-ran-PNonOx29 | 91 | 33 | 15,000 | 1.09 |
| LN19 | PEtOx162-ran-PNonOx38 | 200 | 19 | 29,700 | 1.11 |
| LN29 | PEtOx140-ran-PNonOx57 | 197 | 29 | 28,000 | 1.13 |
2.2. Influence of Nonyl Side Chain Content: Poly(2-ethyl-2-oxazoline)-ran-poly(2-nonyl-2-oxazoline) Containing 20% Nonyl Chains



2.3. Highly Hydrophobic Copolymers: Poly(2-ethyl-2-oxazoline)-ran-poly(2-nonyl-2-oxazoline) Containing 25% and 30% Nonyl Chains

2.4. Poly(2-ethyl-2-oxazoline)-ran-poly(2-nonyl-2-oxazoline): Influence of Polymer Length

3. Experimental Section
3.1. Materials
3.2. Instrumentation
3.3. Turbidimetry and Dynamic Light Scattering Studies
3.4. Poly[(2-ethyl-2-oxazoline)-ran-(2-nonyl-2-oxazoline)] Synthesis
| ID | Target Polymer Composition | Obtained Polymer Composition (a) | DP (Target/Obtained) | MeOTs (g) | EtOx (g) | NonOx (g) | MeCN (mL) |
|---|---|---|---|---|---|---|---|
| SN12 | PEtOx90-ran-PNonOx10 | PEtOx90-ran-PNonOx12 | 100/102 | 0.0335 | 1.606 | 0.355 | 2.491 |
| SN20 | PEtOx80-ran-PNonOx20 | PEtOx84-ran-PNonOx21 | 100/105 | 0.0272 | 1.454 | 0.747 | 2.299 |
| SN25 | PEtOx75-ran-PNonOx25 | PEtOx75-ran-PNonOx25 | 100/100 | 0.0223 | 0.892 | 0.592 | 1.155 |
| SN33 | PEtOx70-ran-PNonOx30 | PEtOx62-ran-PNonOx29 | 100/91 | 0.0272 | 1.272 | 1.120 | 2.108 |
| LN19 | PEtOx160-ran-PNonOx40 | PEtOx162-ran-PNonOx38 | 200/200 | 0.0109 | 0.933 | 0.464 | 1.502 |
| LN29 | PEtOx140-ran-PNonOx60 | PEtOx140-ran-PNonOx57 | 200/197 | 0.0112 | 0.833 | 0.710 | 1.104 |
3.5. Preparation of Poly[(2-ethyl-2-oxazoline)-ran-(2-nonyl-2-oxazoline)] Solutions and Titration
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhang, J.; Pu, G.; Dubay, M.R.; Zhao, Y.; Severtson, S.J. Repositionable pressure-sensitive adhesive possessing thermal-stimuli switchable transparency. J. Mater. Chem. C 2013, 1, 1080–1086. [Google Scholar] [CrossRef]
- Rotzetter, A.C.; Schumacher, C.M.; Bubenhofer, S.B.; Grass, R.N.; Gerber, L.C.; Zeltner, M.; Stark, W.J. Thermoresponsive polymer induced sweating surfaces as an efficient way to passively cool buildings. Adv. Mater. 2012, 24, 5352–5356. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Zhu, H.; Hendrix, M.M.R.M.; Lousberg, N.J.H.G.M.; de With, G.; Esteves, A.C.C.; Xin, J.H. Temperature-triggered collection and release of water from fogs by a sponge-like cotton fabric. Adv. Mater. 2013, 25, 1150–1154. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, A.; Okano, T. Intelligent thermoresponsive polymeric stationary phases for aqueous chromatography of biological compounds. Prog. Polym. Sci. 2002, 27, 1165–1193. [Google Scholar] [CrossRef]
- Tan, I.; Roohi, F.; Titirici, M.-M. Thermoresponsive polymers in liquid chromatography. Anal. Methods 2011, 4, 34–43. [Google Scholar] [CrossRef]
- Defize, T.; Riva, R.; Raquez, J.-M.; Dubois, P.; Jérôme, C.; Alexandre, M. Thermoreversibly crosslinked poly(ε-caprolactone) as recyclable shape-memory polymer network. Macromol. Rapid Commun. 2011, 32, 1264–1269. [Google Scholar] [CrossRef] [PubMed]
- Klouda, L.; Mikos, A.G. Thermoresponsive hydrogels in biomedical applications. Eur. J. Pharm. Biopharm. 2008, 68, 34–45. [Google Scholar] [CrossRef] [PubMed]
- Ward, M.A.; Georgiou, T.K. Thermoresponsive polymers for biomedical applications. Polymers 2011, 3, 1215–1242. [Google Scholar] [CrossRef]
- Kedar, U.; Phutane, P.; Shidhaye, S.; Kadam, V. Advances in polymeric micelles for drug delivery and tumor targeting. Nanomed. NBM 2010, 6, 714–729. [Google Scholar] [CrossRef]
- Brun-Graeppi, A.K.A.S.; Richard, C.; Bessodes, M.; Scherman, D.; Merten, O.-W. Thermoresponsive surfaces for cell culture and enzyme-free cell detachment. Prog. Polym. Sci. 2010, 35, 1311–1324. [Google Scholar] [CrossRef]
- Lee, J.; Kotov, N.A. Thermometer design at the nanoscale. Nano Today 2007, 2, 48–51. [Google Scholar] [CrossRef]
- Gota, C.; Okabe, K.; Funatsu, T.; Harada, Y.; Uchiyama, S. Hydrophilic fluorescent nanogel thermometer for intracellular thermometry. J. Am. Chem. Soc. 2009, 131, 2766–2767. [Google Scholar] [CrossRef] [PubMed]
- McCabe, K.M.; Hernandez, M. Molecular thermometry. Pediatr. Res. 2010, 67, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Pietsch, C.; Schubert, U.S.; Hoogenboom, R. Aqueous polymeric sensors based on temperature-induced polymer phase transitions and solvatochromic dyes. Chem. Commun. 2011, 47, 8750–8765. [Google Scholar] [CrossRef]
- Pietsch, C.; Vollrath, A.; Hoogenboom, R.; Schubert, U.S. A fluorescent thermometer based on a pyrene-labeled thermoresponsive polymer. Sensors 2010, 10, 7979–7990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Okabe, K.; Inada, N.; Gota, C.; Harada, Y.; Funatsu, T.; Uchiyama, S. Intracellular temperature mapping with a fluorescent polymeric thermometer and fluorescence lifetime imaging microscopy. Nat. Commun. 2012, 3, 705. [Google Scholar] [CrossRef] [PubMed]
- Hoogenboom, R. Smart Polymers and their Applications; Aguilar, M.R., Román, J.S., Eds.; Woodhead Publishing: Cambridge, UK, 2014; pp. 15–44. [Google Scholar]
- Lutz, J.-F. Thermo-switchable materials prepared using the OEGMA-platform. Adv. Mater. 2011, 23, 2237–2243. [Google Scholar] [CrossRef]
- Eggenhuisen, T.M.; Becer, C.R.; Fijten, M.W.M.; Eckardt, R.; Hoogenboom, R.; Schubert, U.S. Libraries of statistical hydroxypropyl acrylate containing copolymers with LCST properties prepared by NMP. Macromolecules 2008, 41, 5132–5140. [Google Scholar] [CrossRef]
- Glassner, M.; Lava, K.; de la Rosa, V.R.; Hoogenboom, R. Tuning the LCST of poly(2-cyclopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline. J. Polym. Sci. Part A Polym. Chem. 2014, 52, 3118–3122. [Google Scholar] [CrossRef]
- Huber, S.; Jordan, R. Modulation of the lower critical solution temperature of 2-alkyl-2-oxazoline copolymers. Colloid Polym. Sci. 2008, 286, 395–402. [Google Scholar] [CrossRef]
- Diehl, C.; Schlaad, H. Thermo-responsive polyoxazolines with widely tuneable LCST. Macromol. Biosci. 2009, 9, 157–161. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-S.; Kataoka, K. Comprehensive and accurate control of thermosensitivity of poly(2-alkyl-2-oxazoline)s via well-defined gradient or random copolymerization. Macromolecules 2007, 40, 3599–3609. [Google Scholar] [CrossRef]
- Park, J.-S.; Kataoka, K. Precise control of lower critical solution temperature of thermosensitive poly(2-isopropyl-2-oxazoline) via gradient copolymerization with 2-ethyl-2-oxazoline as a hydrophilic comonomer. Macromolecules 2006, 39, 6622–6630. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Thijs, H.M.L.; Jochems, M.J.H.C.; van Lankvelt, B.M.; Fijten, M.W.M.; Schubert, U.S. Tuning the LCST of poly(2-oxazoline)s by varying composition and molecular weight: Alternatives to poly(N-isopropylacrylamide)? Chem. Commun. 2008, 2008, 5758–5760. [Google Scholar] [CrossRef]
- Fijten, M.W.M.; Kranenburg, J.M.; Thijs, H.M.L.; Paulus, R.M.; van Lankvelt, B.M.; de Hullu, J.; Springintveld, M.; Thielen, D.J.G.; Tweedie, C.A.; Hoogenboom, R.; et al. Synthesis and structure–property relationships of random and block copolymers: A direct comparison for copoly(2-oxazoline)s. Macromolecules 2007, 40, 5879–5886. [Google Scholar] [CrossRef]
- Lambermont-Thijs, H.M. L.; Hoogenboom, R.; Fustin, C.-A.; Bomal-D’Haese, C.; Gohy, J.-F.; Schubert, U.S. Solubility behavior of amphiphilic block and random copolymers based on 2-ethyl-2-oxazoline and 2-nonyl-2-oxazoline in binary water–ethanol mixtures. J. Polym. Sci. Part A Polym. Chem. 2009, 47, 515–522. [Google Scholar] [CrossRef]
- Volet, G.; Chanthavong, V.; Wintgens, V.; Amiel, C. Synthesis of monoalkyl end-capped poly(2-methyl-2-oxazoline) and its micelle formation in aqueous solution. Macromolecules 2005, 38, 5190–5197. [Google Scholar] [CrossRef]
- Ritter, H.; Sadowski, O.; Tepper, E. Influence of cyclodextrin molecules on the synthesis and the thermoresponsive solution behavior of N-isopropylacrylamide copolymers with adamantyl groups in the side-chains. Angew. Chem. Int. Ed. 2003, 42, 3171–3173. [Google Scholar] [CrossRef]
- Schmidt, B.V.K.J.; Hetzer, M.; Ritter, H.; Barner-Kowollik, C. Modulation of the thermoresponsive behavior of poly(N,N-diethylacrylamide) via cyclodextrin host/guest interactions. Macromol. Rapid Commun. 2013, 34, 1306–1311. [Google Scholar] [CrossRef] [PubMed]
- Reinelt, S.; Steinke, D.; Ritter, H. End-group-functionalized poly(N,N-diethylacrylamide) via free-radical chain transfer polymerization: Influence of sulfur oxidation and cyclodextrin on self-organization and cloud points in water. Beilstein J. Org. Chem. 2014, 10, 680–691. [Google Scholar] [CrossRef] [PubMed]
- Burkhart, A.; Ritter, H. Influence of cyclodextrin on the UCST- and LCST-behavior of poly(2-methacrylamido-caprolactam)-co-(N,N-dimethylacrylamide). Beilstein J. Org. Chem. 2014, 10, 1951–1958. [Google Scholar] [CrossRef] [PubMed]
- Sambe, L.; de la Rosa, V.R.; Belal, K.; Stoffelbach, F.; Lyskawa, J.; Delattre, F.; Bria, M.; Cooke, G.; Hoogenboom, R.; Woisel, P. Programmable polymer-based supramolecular temperature sensor with a memory function. Angew. Chem. Int. Ed. 2014, 53, 5044–5048. [Google Scholar]
- Sakai, R.; Otsuka, I.; Satoh, T.; Kakuchi, R.; Kaga, H.; Kakuchi, T. Thermoresponsive on-off switching of chiroptical property induced in poly(4'-ethynylbenzo-15-crown-5)/α-amino acid system. Macromolecules 2006, 39, 4032–4037. [Google Scholar] [CrossRef]
- Yashima, E.; Maeda, K.; Sato, O. Switching of a macromolecular helicity for visual distinction of molecular recognition events. J. Am. Chem. Soc. 2001, 123, 8159–8160. [Google Scholar] [CrossRef] [PubMed]
- Bigot, J.; Charleux, B.; Cooke, G.; Delattre, F.; Fournier, D.; Lyskawa, J.; Sambe, L.; Stoffelbach, F.; Woisel, P. Tetrathiafulvalene end-functionalized poly(N-isopropylacrylamide): A new class of amphiphilic polymer for the creation of multistimuli responsive micelles. J. Am. Chem. Soc. 2010, 132, 10796–10801. [Google Scholar] [CrossRef] [PubMed]
- Sambe, L.; Stoffelbach, F.; Lyskawa, J.; Delattre, F.; Fournier, D.; Bouteiller, L.; Charleux, B.; Cooke, G.; Woisel, P. Host–guest modulation of the micellization of a tetrathiafulvalene-functionalized poly(N-isopropylacrylamide). Macromolecules 2011, 44, 6532–6538. [Google Scholar] [CrossRef]
- Chi, X.; Ji, X.; Xia, D.; Huang, F. A Dual-responsive supra-amphiphilic polypseudorotaxane constructed from a water-soluble pillar[7]arene and an azobenzene-containing random copolymer. J. Am. Chem. Soc. 2015, 137, 1440–1443. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Jie, K.; Huang, F. Supramolecular amphiphiles based on host–guest molecular recognition motifs. Chem. Rev. 2015. [Google Scholar] [CrossRef]
- Ji, X.; Li, J.; Chen, J.; Chi, X.; Zhu, K.; Yan, X.; Zhang, M.; Huang, F. Supramolecular micelles constructed by crown ether-based molecular recognition. Macromolecules 2012, 45, 6457–6463. [Google Scholar] [CrossRef]
- Ji, X.; Dong, S.; Wei, P.; Xia, D.; Huang, F. A novel diblock copolymer with a supramolecular polymer block and a traditional polymer block: Preparation, controllable self-assembly in water, and application in controlled release. Adv. Mater. 2013, 25, 5725–5729. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, V.R.; Nau, W.M.; Hoogenboom, R. Tuning temperature responsive poly(2-alkyl-2-oxazoline)s by supramolecular host–guest interactions. Org. Biomol. Chem. 2015, 13, 3048–3057. [Google Scholar] [CrossRef] [PubMed]
- De la Rosa, V.R.; Hoogenboom, R. Solution polymeric optical temperature sensors with long-term memory function powered by supramolecular chemistry. Chem. Eur. J. 2015, 21, 1302–1311. [Google Scholar] [CrossRef] [PubMed]
- Glassner, M.; D’hooge, D.R.; Park, J.Y.; van Steenberge, P.H.M.; Monnery, B.D.; Reyniers, M.-F.; Hoogenboom, R. Systematic investigation of alkyl sulfonate initiators for the cationic ring-opening polymerization of 2-oxazolines revealing optimal combinations of monomers and initiators. Eur. Polym. J. 2015, 65, 298–304. [Google Scholar] [CrossRef]
- Hoogenboom, R.; Fijten, M.W.M.; Wijnans, S.; van den Berg, A.M.J.; Thijs, H.M.L.; Schubert, U.S. High-throughput synthesis and screening of a library of random and gradient copoly(2-oxazoline)s. J. Comb. Chem. 2006, 8, 145–148. [Google Scholar] [CrossRef] [PubMed]
- Litt, M.; Levy, A.; Herz, J. Polymerization of cyclic imino ethers. X. Kinetics, chain transfer, and repolymerization. J. Macromol. Sci. Chem. 1975, 9, 703–727. [Google Scholar] [CrossRef]
- Lagona, J.; Mukhopadhyay, P.; Chakrabarti, S.; Isaacs, L. The cucurbit[n]uril family. Angew. Chem. Int. Ed. 2005, 44, 4844–4870. [Google Scholar] [CrossRef]
- Szejtli, J. Introduction and general overview of cyclodextrin chemistry. Chem. Rev. 1998, 98, 1743–1754. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Jung, I.-S.; Kim, S.-Y.; Lee, E.; Kang, J.-K.; Sakamoto, S.; Yamaguchi, K.; Kim, K. New cucurbituril homologues: Syntheses, isolation, characterization, and X-ray crystal structures of cucurbit[n]uril (n = 5, 7, and 8). J. Am. Chem. Soc. 2000, 122, 540–541. [Google Scholar] [CrossRef]
- Day, A.; Arnold, A.P.; Blanch, R.J.; Snushall, B. Controlling factors in the synthesis of cucurbituril and its homologues. J. Org. Chem. 2001, 66, 8094–8100. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
R. de la Rosa, V.; Nau, W.M.; Hoogenboom, R. Thermoresponsive Interplay of Water Insoluble Poly(2-alkyl-2-oxazoline)s Composition and Supramolecular Host–Guest Interactions. Int. J. Mol. Sci. 2015, 16, 7428-7444. https://doi.org/10.3390/ijms16047428
R. de la Rosa V, Nau WM, Hoogenboom R. Thermoresponsive Interplay of Water Insoluble Poly(2-alkyl-2-oxazoline)s Composition and Supramolecular Host–Guest Interactions. International Journal of Molecular Sciences. 2015; 16(4):7428-7444. https://doi.org/10.3390/ijms16047428
Chicago/Turabian StyleR. de la Rosa, Victor, Werner M. Nau, and Richard Hoogenboom. 2015. "Thermoresponsive Interplay of Water Insoluble Poly(2-alkyl-2-oxazoline)s Composition and Supramolecular Host–Guest Interactions" International Journal of Molecular Sciences 16, no. 4: 7428-7444. https://doi.org/10.3390/ijms16047428