Hydrogels for Engineering of Perfusable Vascular Networks
Abstract
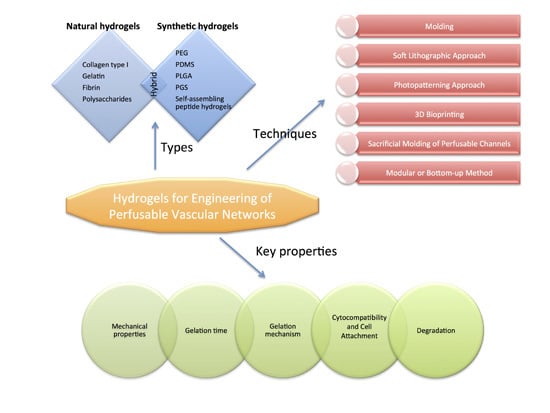
:1. Introduction
2. Hydrogels Used as Materials for Vascular Network Fabrication
2.1. Natural Hydrogels
2.2. Synthetic Hydrogels
2.3. Hybrid Hydrogels
3. Key Hydrogel Properties for Vascular Engineering
3.1. Mechanical Properties

3.2. Gelation Time
3.3. Gelation Mechanisms
3.4. Cytocompatibility and Cell Attachment
3.5. Degradation
4. Methods for Vascular Network Scaffold Fabrication
4.1. Molding

4.2. Soft Lithographic Approach

4.3. Photopatterning Approach

4.4. 3D Bioprinting

4.5. Sacrificial Molding of Perfusable Channels
4.6. Modular or Bottom-up Method

5. Future Perspectives
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Khademhosseini, A.; Vacanti, J.; Langer, R. Progress in tissue engineering. Sci. Am. 2009, 300, 64–71. [Google Scholar] [CrossRef] [PubMed]
- Carmeliet, P.; Jain, R.K. Angiogenesis in cancer and other diseases. Nature 2000, 407, 249–257. [Google Scholar] [CrossRef] [PubMed]
- Malda, J.; Klein, T.J.; Upton, Z. The roles of hypoxia in the in vitro engineering of tissues. Tissue Eng. 2007, 13, 2153–2162. [Google Scholar] [CrossRef] [PubMed]
- Menolascina, F.; Bellomo, D.; Maiwald, T.; Bevilacqua, V.; Ciminelli, C.; Paradiso, A.; Tommasi, S. Developing optimal input design strategies in cancer systems biology with applications to microfluidic device engineering. BMC Bioinform. 2009, 10 (Suppl. 12), S4. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.S.; Stevens, K.R.; Yang, M.T.; Baker, B.M.; Nguyen, D.H.; Cohen, D.M.; Toro, E.; Chen, A.A.; Galie, P.A.; Yu, X.; et al. Rapid casting of patterned vascular networks for perfusable engineered three-dimensional tissues. Nat. Mater. 2012, 11, 768–774. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Chen, J.; Craven, M.; Choi, N.W.; Totorica, S.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; Fischbach-Teschl, C.; López, J.A.; et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9342–9347. [Google Scholar] [CrossRef] [PubMed]
- Takei, T.; Sakai, S.; Ono, T.; Ijima, H.; Kawakami, K. Fabrication of endothelialized tube in collagen gel as starting point for self-developing capillary-like network to construct three-dimensional organs in vitro. Biotechnol. Bioeng. 2006, 95, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Marchant, R.E. Design properties of hydrogel tissue-engineering scaffolds. Expert Rev. Med. Devices 2011, 8, 607–626. [Google Scholar] [CrossRef] [PubMed]
- Kopecek, J. Hydrogel biomaterials: A smart future? Biomaterials 2007, 28, 5185–5192. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P. Biomaterials: Spotlight on hydrogels. Nat. Mater. 2009, 8, 451–453. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.Q.; Tay, R.; Khan, M.; Ee, P.L.R.; Hedrick, J.L.; Yang, Y.Y. Synthetic hydrogels for controlled stem cell differentiation. Soft Matter 2010, 6, 67–81. [Google Scholar] [CrossRef]
- Varghese, S.; Elisseeff, J.H. Hydrogels for musculoskeletal tissue engineering. Adv. Polym. Sci. 2006, 203, 95–144. [Google Scholar]
- Cushing, M.C.; Anseth, K.S. Materials science. Hydrogel cell culture. Science 2007, 316, 1133–1134. [Google Scholar] [CrossRef] [PubMed]
- Nicodemus, G.D.; Bryant, S.J. Cell encapsulation in biodegradable hydrogels for tissue engineering applications. Tissue Eng. B Rev. 2008, 14, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.; Truslow, J.; Tien, J. The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010, 31, 4706–4714. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, F.L.; Gagnieu, C.H. Denatured thiolated collagen. II. Cross-linking by oxidation. Biomaterials 1997, 18, 815–821. [Google Scholar] [CrossRef]
- Sakai, S.; Hirose, K.; Taguchi, K.; Ogushi, Y.; Kawakami, K. An injectable, in situ enzymatically gellable, gelatin derivative for drug delivery and tissue engineering. Biomaterials 2009, 30, 3371–3377. [Google Scholar] [CrossRef] [PubMed]
- Paguirigan, A.; Beebe, D.J. Gelatin based microfluidic devices for cell culture. Lab Chip 2006, 6, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Ravi, S.; Chaikof, E.L. Biomaterials for vascular tissue engineering. Regen. Med. 2010, 5, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Wang, X. Fluid and cell behaviors along a 3D printed alginate/gelatin/fibrin channel. Biotechnol. Bioeng. 2015, 112, 1683–1695. [Google Scholar] [CrossRef] [PubMed]
- Swartz, D.D.; Russell, J.A.; Andreadis, S.T. Engineering of fibrin-based functional and implantable small-diameter blood vessels. Am. J. Physiol. Heart Circ. Physiol. 2005, 288, H1451–H1460. [Google Scholar] [CrossRef] [PubMed]
- Ramamurthi, A.; Vesely, I. Ultraviolet light-induced modification of crosslinked hyaluronan gels. J. Biomed. Mater. Res. A 2003, 66, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.K.; Ma, P.X. Ionically crosslinked alginate hydrogels as scaffolds for tissue engineering: Part I. Structure, gelation rate and mechanical properties. Biomaterials 2001, 22, 511–521. [Google Scholar] [CrossRef]
- Kim, I.Y.; Seo, S.J.; Moon, H.S.; Yoo, M.K.; Park, I.Y.; Kim, B.C.; Cho, C.S. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008, 26, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Ling, Y.; Rubin, J.; Deng, Y.; Huang, C.; Demirci, U.; Karp, J.M.; Khademhosseini, A. A cell-laden microfluidic hydrogel. Lab Chip 2007, 7, 756–762. [Google Scholar] [CrossRef] [PubMed]
- Choi, N.W.; Cabodi, M.; Held, B.; Gleghorn, J.P.; Bonassar, L.J.; Stroock, A.D. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007, 6, 908–915. [Google Scholar] [CrossRef] [PubMed]
- Davidenko, N.; Campbell, J.J.; Thian, E.S.; Watson, C.J.; Cameron, R.E. Collagen-hyaluronic acid scaffolds for adipose tissue engineering. Acta Biomater. 2010, 6, 3957–3968. [Google Scholar] [CrossRef] [PubMed]
- Dhandayuthapani, B.; Krishnan, U.M.; Sethuraman, S. Fabrication and characterization of chitosan-gelatin blend nanofiber for skin tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2010, 94, 264–272. [Google Scholar] [CrossRef] [PubMed]
- Shikanov, A.; Xu, M.; Woodruff, T.K.; Shea, L.D. Interpenetrating fibrin-alginate matrices for in vitro ovarian follicle development. Biomaterials 2009, 30, 5476–5485. [Google Scholar] [CrossRef] [PubMed]
- Cuchiara, M.P.; Allen, A.C.; Chen, T.M. Multilayer microfluidic PEGDA hydrogels. Biomaterials 2010, 31, 5491–5497. [Google Scholar] [CrossRef] [PubMed]
- Chen, R.; Hunt, J.A. Biomimetic materials processing for tissue engineering processes. J. Mater. Chem. 2007, 17, 3974–3979. [Google Scholar] [CrossRef]
- Wan, J. Microfluidic-based synthesis of hydrogel particles for cell microencapsulation and cell-based drug delivery. Polymers 2012, 4, 1084–1108. [Google Scholar] [CrossRef]
- Langer, R.; Tirrell, D.A. Designing materials for biology and medicine. Nature 2004, 428, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Borenstein, J.T.; Tupper, M.M.; Mack, P.J.; Weinberg, E.J.; Khalil, A.S.; Hsiao, J.; Garcia-Cardena, G. Functional endothelialized microvascular networks with circular cross-sections in a tissue culture substrate. Biomed. Microdevices 2010, 12, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Fiddes, L.K.; Raz, N.; Srigunapalan, S.; Tumarkan, E.; Simmons, C.A.; Wheeler, A.R.; Kumacheva, E. A circular cross-section PDMS microfluidics system for replication of cardiovascular flow conditions. Biomaterials 2010, 31, 3459–3464. [Google Scholar] [CrossRef] [PubMed]
- McUsic, A.C.; Lamba, D.A.; Reh, T.A. Guiding the morphogenesis of dissociated newborn mouse retinal cells and hES cell-derived retinal cells by soft lithography-patterned microchannel PLGA scaffolds. Biomaterials 2012, 33, 1396–1405. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Lu, L.; Kolewe, M.E.; Park, H.; Larson, B.L.; Kim, E.S.; Freed, L.E. A biodegradable microvessel scaffold as a framework to enable vascular support of engineered tissues. Biomaterials 2013, 34, 10007–10015. [Google Scholar] [CrossRef] [PubMed]
- Bettinger, C.J.; Weinberg, E.J.; Kulig, K.M.; Vacanti, J.P.; Wang, Y.; Borenstein, J.T.; Langer, R. Three-dimensional microfluidic tissue-engineering scaffolds using a flexible biodegradable polymer. Adv. Mater. 2005, 18, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Buxton, A.N.; Zhu, J.; Marchant, R.E.; West, J.L.; Yoo, J.U.; Johnstone, B. Design and characterization of poly(ethylene glycol) photopolymerizable semi-interpenetrating networks for chondrogenesis of human mesenchymal stem cells. Tissue Eng. 2007, 13, 2549–2560. [Google Scholar] [CrossRef] [PubMed]
- Beamish, J.A.; Zhu, J.; Kottke-Marchant, K.; Marchant, R.E. The effects of monoacrylate poly(ethylene glycol) on the properties of poly(ethylene glycol) diacrylate hydrogels used for tissue engineering. J. Biomed. Mater. Res. A 2010, 92, 441–450. [Google Scholar] [PubMed]
- Liu Tsang, V.; Chen, A.A.; Cho, L.M.; Jadin, K.D.; Sah, R.L.; DeLong, S.; West, J.L.; Bhatia, S.N. Fabrication of 3D hepatic tissues by additive photopatterning of cellular hydrogels. FASEB J. 2007, 21, 790–801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J. Bioactive modification of poly(ethylene glycol) hydrogels for tissue engineering. Biomaterials 2010, 31, 4639–4656. [Google Scholar] [CrossRef] [PubMed]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [PubMed]
- Sarig-Nadir, O.; Livnat, N.; Zajdman, R.; Shoham, S.; Seliktar, D. Laser photoablation of guidance microchannels into hydrogels directs cell growth in three dimensions. Biophys. J. 2009, 96, 4743–4752. [Google Scholar] [CrossRef] [PubMed]
- Hauser, C.A.; Zhang, S. Designer self-assembling peptide nanofiber biological materials. Chem. Soc. Rev. 2010, 39, 2780–2790. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Xiao, J.; Dang, F. Surface modification of poly(dimethylsiloxane) using ionic complementary peptides to minimize nonspecific protein adsorption. Langmuir 2015, 31, 5891–588. [Google Scholar] [CrossRef] [PubMed]
- Goktas, M.; Cinar, G.; Orujalipoor, I.; Die, S.; Tekinay, A.B.; Guler, M.O. Self-assembled peptide amphiphile nanofibers and PEG composite hydrogels as tunable ECM mimetic microenvironment. Biomacromolecules 2015, 16, 1247–1258. [Google Scholar] [CrossRef] [PubMed]
- Potts, J.R.; Campbell, I.D. Fibronectin structure and assembly. Curr. Opin. Cell Biol. 1994, 6, 648–655. [Google Scholar] [CrossRef]
- Shalumon, K.T.; Deepthi, S.; Anupama, M.S.; Nair, S.V.; Jayakumar, R.; Chennazhi, K.P. Fabrication of poly(l-lactic acid)/gelatin composite tubular scaffolds for vascular engineering. Int. J. Biol. Macromol. 2015, 72, 1048–1055. [Google Scholar] [CrossRef] [PubMed]
- Kageyama, T.; Kakegawa, T.; Osaki, T. Rapid engineering of endothelial cell-lined vascular-like structures in in situ crosslinkable hydrogels. Biofabrication 2014, 6, 025006. [Google Scholar] [CrossRef] [PubMed]
- Van den Bulcke, A.I.; Bogdanov, B.; de Rooze, N.; Schacht, E.H.; Cornelissen, M.; Berghmans, H. Structural and rheological properties of methacrylamide modified gelatin hydrogels. Biomacromolecules 2000, 1, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Nichol, J.W.; Koshy, S.T.; Bae, H.; Hwang, C.M.; Yamanlar, S.; Khademhosseini, A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials 2010, 31, 5536–5544. [Google Scholar] [CrossRef] [PubMed]
- Arda, K.; Ciledag, N.; Aktas, E.; Aribas, B.K.; Köse, K. Quantitative assessment of normal soft-tissue elasticity using shear-wave ultrasound elastography. Am. J. Roentgenol. 2011, 197, 532–536. [Google Scholar] [CrossRef] [PubMed]
- Achterberg, V.F.; Buscemi, L.; Diekmann, H.; Smith-Clerc, J.; Schwengler, H.; Meister, J.J.; Wenck, H.; Gallinat, S.; Hinz, B. The nano-scale mechanical properties of the extracellular matrix regulate dermal fibroblast function. J. Investig. Dermatol. 2014, 134, 1862–1872. [Google Scholar] [CrossRef] [PubMed]
- Hoenig, E.; Leicht, U.; Winkler, T.; Mielke, G.; Beck, K.; Peters, F.; Schilling, A.F.; Morlock, M.M. Mechanical properties of native and tissue-engineered cartilage depend oncarrier permeability: A bioreactor study. Tissue Eng. A 2013, 19, 1534–1542. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix elasticity directs stem cell lineage specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [PubMed]
- Geckil, H.; Xu, F.; Zhang, X.; Moon, S.; Demirci, U. Engineering hydrogels as extracellular matrix mimics. Nanomedicine (Lond.) 2010, 5, 469–484. [Google Scholar] [CrossRef] [PubMed]
- Burdick, J.A.; Prestwich, G.D. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 2011, 23, H41–H56. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, P.; Bausch, A.R. The compaction of gels by cells: A case of collective mechanical activity. Integr. Biol. (Camb.) 2009, 1, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Bryant, S.J.; Bender, R.J.; Durand, K.L.; Anseth, K.S. Encapsulating chondrocytes in degrading PEG hydrogels with high modulus: engineering gel structural changes to facilitate cartilaginous tissue production. Biotechnol. Bioeng. 2004, 86, 747–755. [Google Scholar] [CrossRef] [PubMed]
- Huang, G.; Wang, L.; Wang, S.; Han, Y.; Wu, J.; Zhang, Q.; Xu, F.; Lu, T.J. Engineering three-dimensional cell mechanical microenvironment with hydrogels. Biofabrication 2012, 4, 042001. [Google Scholar] [CrossRef] [PubMed]
- Hwang, C.M.; Sant, S.; Masaeli, M.; Kachouie, N.N.; Zamanian, B.; Lee, S.H.; Khademhosseini, A. Fabrication of three-dimensional porous cell-laden hydrogel for tissue engineering. Biofabrication 2010, 2, 035003. [Google Scholar] [CrossRef] [PubMed]
- Kuboki, Y.; Takita, H.; Kobayashi, D.; Tsuruga, E.; Inoue, M.; Murata, M.; Nagai, N.; Dohi, Y.; Ohgushi, H. BMP-induced osteogenesis on the surface of hydroxyapatite with geometrically feasible and nonfeasible structures: Topology of osteogenesis. J. Biomed. Mater. Res. 1998, 39, 190–199. [Google Scholar] [CrossRef]
- Itala, A.I.; Ylanen, H.O.; Ekholm, C.; Karlsson, K.H.; Aro, H.T. Pore diameter of more than 100 μm is not requisite for bone in growth in rabbits. J. Biomed. Mater. Res. 2001, 58, 679–683. [Google Scholar] [CrossRef] [PubMed]
- Karageorgiou, V.; Kaplan, D. Porosity of 3D biomaterial scaffolds and osteogenesis. Biomaterials 2005, 26, 5474–5491. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Kim, S.W.; Lee, D.S. In situ gelling stimuli-sensitive block copolymer hydrogels for drug delivery. J. Control. Release 2008, 127, 189–207. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.P.; Bruzewicz, D.A.; Glavan, A.; Butte, M.J.; Whitesides, G.M. Cell encapsulation in sub-mm sized gel modules using replica molding. PLoS ONE 2008, 3, e2258. [Google Scholar] [CrossRef] [PubMed]
- Kisiday, J.; Jin, M.; Kurz, B.; Hung, H.; Semino, C.; Zhang, S.; Grodzinsky, A.J. Self-assembling peptide hydrogel fosters chondrocyte extracellular matrix production and cell division: Implication for cartilage tissue repair. Proc. Natl. Acad. Sci. USA 2002, 99, 9996–10001. [Google Scholar] [CrossRef] [PubMed]
- Slaughter, B.V.; Khurshid, S.S.; Fisher, O.Z.; Khademhosseini, A.; Peppas, N.A. Hydrogels in regenerative medicine. Adv. Mater. 2009, 21, 3307–3329. [Google Scholar] [CrossRef] [PubMed]
- Fisher, J.P.; Mikos, A.G.; Bronzino, O.D.; Peterson, D.R. Tissue Engineering: Principles and Practices, 1st ed.; CRC Press: Boca Raton, FL, USA, 2012; pp. 2010–2011. [Google Scholar]
- Yung, C.W.; Wu, L.Q.; Tullman, J.A.; Payne, G.F.; Bentley, W.E.; Barbari, T.A. Transglutaminase crosslinked gelatin as a tissue engineering scaffold. J. Biomed. Mater. Res. A 2007, 83, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Rhodes, J.M.; Simons, M. The extracellular matrix and blood vessel formation; not just a scaffold. J. Cell. Mol. Med. 2007, 11, 176–205. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; McCoard, L.; Xu, X.; Oottamasathien, S.; Prestwich, G.D. Photocrosslinkable hyaluronan-gelatin hydrogels for two-step bioprinting. Tissue Eng. A 2010, 16, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Hern, D.L.; Hubbell, J.A. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J. Biomed. Mater. Res. 1998, 39, 266–276. [Google Scholar] [CrossRef]
- Gasperini, L.; Mano, J.F.; Reis, R.L. Natural polymers for the microencapsulation of cells. J. R. Soc. Interface 2014, 11, 20140817. [Google Scholar] [CrossRef] [PubMed]
- Price, G.M.; Chrobak, K.M.; Tien, J. Effect of cyclic AMP on barrier function of human lymphatic microvascular tubes. Microvasc. Res. 2008, 76, 46–51. [Google Scholar] [CrossRef] [PubMed]
- Price, G.M.; Wong, K.H.; Truslow, J.G.; Leung, A.D.; Acharya, C.; Tien, J. Effect of mechanical factors on the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010, 31, 6182–6189. [Google Scholar] [CrossRef] [PubMed]
- Paguirigan, A.L.; Beebe, D.J. Protocol for the fabrication of enzymatically crosslinked gelatin microchannels for microfluidic cell culture. Nat. Protoc. 2007, 2, 1782–1788. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Ghodousi, M.; Qi, H.; Haas, N.; Xiao, W.; Khademhosseini, A. Sequential assembly of cell-laden hydrogel constructs to engineer vascular-like microchannels. Biotechnol. Bioeng. 2011, 108, 1693–1703. [Google Scholar] [CrossRef] [PubMed]
- Tocchio, A.; Tamplenizza, M.; Martello, F.; Gerges, I.; Rossi, E.; Argentiere, S.; Rodighiero, S.; Zhao, W.; Milani, P.; Lenardi, C. Versatile fabrication of vascularizable scaffolds for large tissue engineering in bioreactor. Biomaterials 2015, 45, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Mironov, V.; Visconti, R.P.; Kasyanov, V.; Forgacs, G.; Drake, C.J.; Markwald, R.R. Organ printing: Tissue spheroids as building blocks. Biomaterials 2009, 30, 2164–2174. [Google Scholar] [CrossRef] [PubMed]
- Lee, V.K.; Kim, D.Y.; Ngo, H.; Lee, Y.; Seo, L.; Yoo, S.S.; Vincent, P.A.; Dai, G. Creating perfused functional vascular channels using 3D bio-printing technology. Biomaterials 2014, 35, 8092–8102. [Google Scholar] [CrossRef] [PubMed]
- Skardal, A.; Zhang, J.; Prestwich, G.D. Bioprinting vessel-like constructs using hyaluronan hydrogels crosslinked with tetrahedral polyethylene glycol tetracrylates. Biomaterials 2010, 31, 6173–6181. [Google Scholar] [CrossRef] [PubMed]
- Serban, M.A.; Prestwich, G.D. Modular extracellular matrices: Solutions for the puzzle. Methods 2008, 45, 93–98. [Google Scholar] [CrossRef] [PubMed]
- Therriault, D.; White, S.R.; Lewis, J.A. Chaotic mixing in three-dimensional microvascular networks fabricated by direct-write assembly. Nat. Mater. 2003, 2, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Wu, W.; Hansen, C.J.; Aragón, A.M.; Geubelle, P.H.; White, S.R.; Lewis, J.A. Direct-write assembly of biomimetic microvascular networks for efficient fluid transport. Soft Matter 2010, 6, 739–742. [Google Scholar] [CrossRef]
- Nazhat, S.N.; Neel, E.A.; Kidane, A.; Ahmed, I.; Hope, C.; Kershaw, M.; Lee, P.D.; Stride, E.; Saffari, N.; Knowles, J.C.; et al. Controlled microchannelling in dense collagen scaffolds by soluble phosphate glass fibers. Biomacromolecules 2007, 8, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Golden, A.P.; Tien, J. Fabrication of microfluidic hydrogels using molded gelatin as a sacrificial element. Lab Chip 2007, 7, 720–725. [Google Scholar] [CrossRef] [PubMed]
- Madden, L.R.; Mortisen, D.J.; Sussman, E.M.; Dupras, S.K.; Fugate, J.A.; Cuy, J.L.; Hauch, K.D.; Laflamme, M.A.; Murry, C.E.; Ratner, B.D. Proangiogenic scaffolds as functional templates for cardiac tissue engineering. Proc. Natl. Acad. Sci. USA 2010, 107, 15211–15216. [Google Scholar] [CrossRef] [PubMed]
- Khademhosseini, A.; Langer, R. Microengineered hydrogels for tissue engineering. Biomaterials 2007, 28, 5087–5092. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.P.; Sefton, M.V. Design and fabrication of sub-mm-sized modules containing encapsulated cells for modular tissue engineering. Tissue Eng. 2007, 13, 1069–1078. [Google Scholar] [CrossRef] [PubMed]
- McGuigan, A.P.; Sefton, M.V. Modular tissue engineering: Fabrication of a gelatin-based construct. J. Tissue Eng. Regen. Med. 2007, 1, 136–145. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Zheng, H.; Poh, P.S.P.; Machens, H.-G.; Schilling, A.F. Hydrogels for Engineering of Perfusable Vascular Networks. Int. J. Mol. Sci. 2015, 16, 15997-16016. https://doi.org/10.3390/ijms160715997
Liu J, Zheng H, Poh PSP, Machens H-G, Schilling AF. Hydrogels for Engineering of Perfusable Vascular Networks. International Journal of Molecular Sciences. 2015; 16(7):15997-16016. https://doi.org/10.3390/ijms160715997
Chicago/Turabian StyleLiu, Juan, Huaiyuan Zheng, Patrina S. P. Poh, Hans-Günther Machens, and Arndt F. Schilling. 2015. "Hydrogels for Engineering of Perfusable Vascular Networks" International Journal of Molecular Sciences 16, no. 7: 15997-16016. https://doi.org/10.3390/ijms160715997
APA StyleLiu, J., Zheng, H., Poh, P. S. P., Machens, H. -G., & Schilling, A. F. (2015). Hydrogels for Engineering of Perfusable Vascular Networks. International Journal of Molecular Sciences, 16(7), 15997-16016. https://doi.org/10.3390/ijms160715997