Cdc42-Interacting Protein 4 Represses E-Cadherin Expression by Promoting β-Catenin Translocation to the Nucleus in Murine Renal Tubular Epithelial Cells
Abstract
:1. Introduction
2. Results
2.1. Distribution and Expression of CIP4 in Renal Tissue of 5/6 Nephrectomized Rats

2.2. TGF-β1 Increases Expression of CIP4 and Induces CIP4 and β-Catenin Translocation to the Nucleus in the NRK-52E Cell Line


2.3. CIP4 Interacts with β-Catenin and Overexpression of CIP4 Induces E-Cadherin Downregulation in NRK-52E Cells

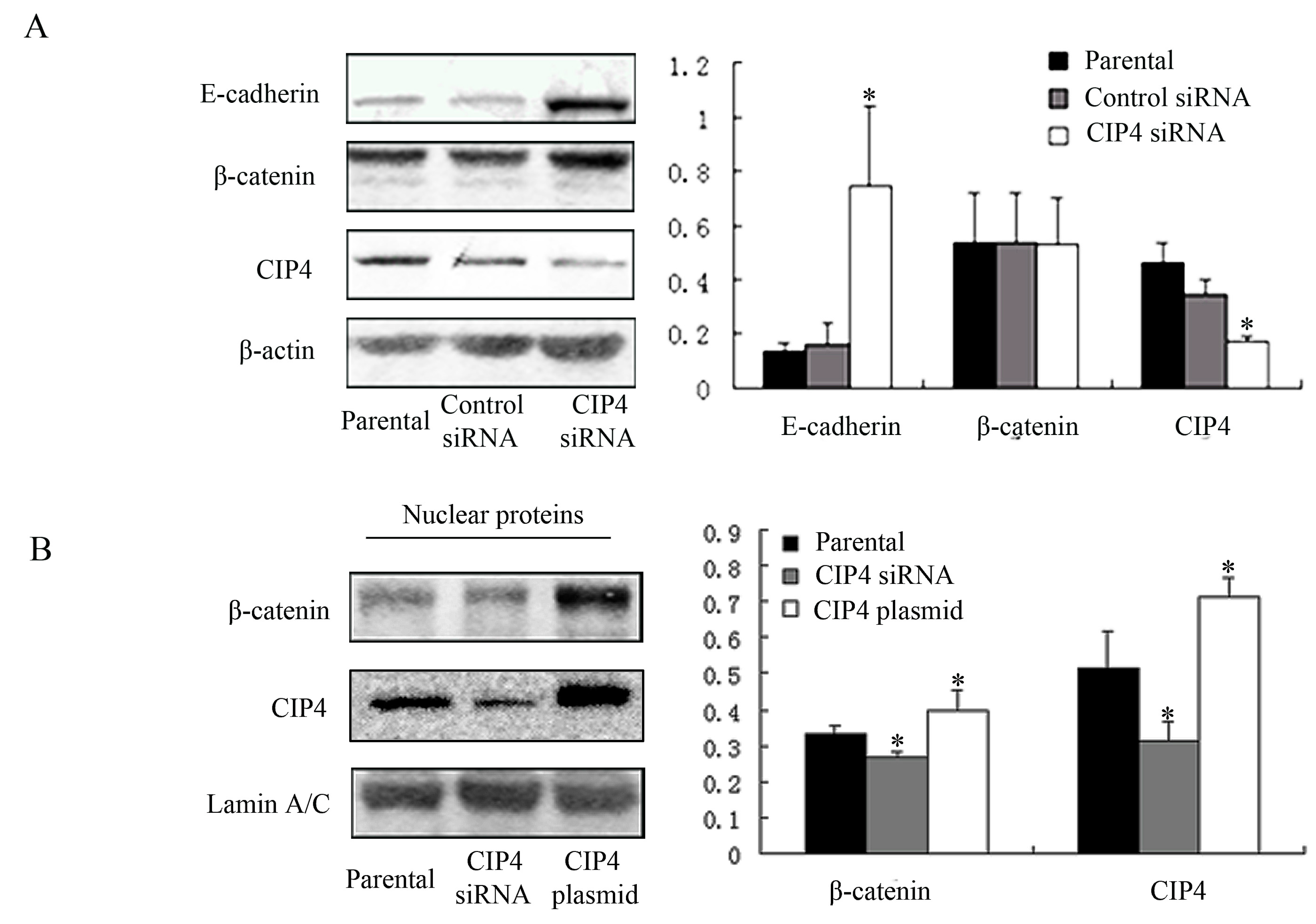
2.4. CIP4 Depletion Reverses the Decreased Expression of E-Cadherin and Regulates Translocation of β-Catenin to the Nucleus of NRK-52E Cells
2.5. β-Catenin Depletion in Cells Overexpressing CIP4 Restores the Expression of E-Cadherin

3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Experimental Protocols
4.3. Cell Culture and Treatment
4.4. Western Blot Analysis
4.5. Establishment of Stable CIP4-Overexpressing NRK-52E Clones
4.6. RNA Interference
4.7. Immunoprecipitation
4.8. Immunohistochemistry
4.9. Statistical Analyses
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Aspenstrom, P. A Cdc42 target protein with homology to the non-kinase domain of FER has a potential role in regulating the actin cytoskeleton. Curr. Biol. 1997, 7, 479–487. [Google Scholar] [CrossRef]
- Tsuji, E.; Tsuji, Y.; Fujiwara, T.; Ogata, S.; Tsukamoto, K.; Saku, K. Splicing variant of Cdc42 interacting protein-4 disrupts beta-catenin-mediated cell–cell adhesion: Expression and function in renal cell carcinoma. Biochem. Biophys. Res. Commun. 2006, 339, 1083–1088. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, P.P.; Pandey, R.; Zheng, R.; Suhoski, M.M.; Monaco-Shawver, L.; Orange, J.S. Cdc42-interacting protein-4 functionally links actin and microtubule networks at the cytolytic NK cell immunological synapse. J. Exp. Med. 2007, 204, 2305–2320. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.E. Non-Smad pathways in TGF-β signaling. Cell Res. 2009, 19, 128–139. [Google Scholar] [CrossRef] [PubMed]
- Kardassis, D.; Murphy, C.; Fotsis, T.; Moustakas, A.; Stournaras, C. Control of transforming growth factor β signal transduction by small GTPases. FEBS J. 2009, 276, 2947–2965. [Google Scholar] [CrossRef] [PubMed]
- Miyazono, K. Transforming growth factor-β signaling in epithelial-mesenchymal transition and progression of cancer. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2009, 85, 314–323. [Google Scholar] [CrossRef] [PubMed]
- Tian, X.; Liu, Z.; Niu, B.; Zhang, J.; Tan, T.K.; Lee, S.R.; Zhao, Y.; Harris, D.C.; Zheng, G. E-cadherin/β-catenin complex and the epithelial barrier. J. Biomed. Biotechnol. 2011. [Google Scholar] [CrossRef] [PubMed]
- Meng, W.; Takeichi, M. Adherens junction: Molecular architecture and regulation. Cold Spring Harb. Perspect. Biol. 2009, 1. [Google Scholar] [CrossRef] [PubMed]
- Baum, B.; Georgiou, M. Dynamics of adherens junctions in epithelial establishment, maintenance, and remodeling. J. Cell Biol. 2011, 192, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Niessen, C.M.; Leckband, D.; Yap, A.S. Tissue organization by cadherin adhesion molecules: Dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiol. Rev. 2011, 91, 691–731. [Google Scholar] [CrossRef] [PubMed]
- Santibanez, J.F.; Quintanilla, M.; Bernabeu, C. TGF-β /TGF-β receptor system and its role in physiological and pathological conditions. Clin. Sci. 2011, 121, 233–251. [Google Scholar] [CrossRef] [PubMed]
- MacDonald, B.T.; Tamai, K.; He, X. Wnt/β-catenin signaling: Components, mechanisms, and diseases. Dev. Cell 2009, 17, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Nelson, W.J.; Nusse, R. Convergence of Wnt, β-catenin, and cadherin pathways. Science 2004, 303, 1483–1487. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y. New insights into epithelial-mesenchymal transition in kidney fibrosis. J. Am. Soc. Nephrol. 2010, 21, 212–222. [Google Scholar] [CrossRef] [PubMed]
- Truesdell, P.; Ahn, J.; Chander, H.; Meens, J.; Watt, K.; Yang, X.; Craig, A.W. CIP4 promotes lung adenocarcinoma metastasis and is associated with poor prognosis. Oncogene 2014, 34, 3527–3535. [Google Scholar] [CrossRef] [PubMed]
- McCrea, P.D.; Gu, D. The catenin family at a glance. J. Cell Sci. 2010, 123, 637–642. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Han, M.; Luo, Y.; Li, C.; Pei, G.; Liao, W.; Bai, S.; Ge, S.; Liu, X.; Xu, G. Role of Sema4C in TGF-β1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. Nephrol. Dial. Transplant. 2011, 26, 1149–1156. [Google Scholar] [CrossRef] [PubMed]
- Zeng, R.; Yao, Y.; Han, M.; Zhao, X.; Liu, X.C.; Wei, J.; Luo, Y.; Zhang, J.; Zhou, J.; Wang, S.; et al. Biliverdin reductase mediates hypoxia-induced EMT via PI3-kinase and Akt. J. Am. Soc. Nephrol. 2008, 19, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Galichon, P.; Finianos, S.; Hertig, A. EMT-MET in renal disease: Should we curb our enthusiasm. Cancer Lett. 2013, 341, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.D. Apico-basal polarity in polycystic kidney disease epithelia. Biochim. Biophys. Acta 2011, 1812, 1239–1248. [Google Scholar] [CrossRef] [PubMed]
- Hills, C.E.; Squires, P.E. TGF-β1-induced epithelial-to-mesenchymal transition and therapeutic intervention in diabetic nephropathy. Am. J. Nephrol. 2010, 31, 68–74. [Google Scholar] [CrossRef] [PubMed]
- Lilien, J.; Balsamo, J. The regulation of cadherin-mediated adhesion by tyrosine phosphorylation/dephosphorylation of β-catenin. Curr. Opin. Cell Biol. 2005, 17, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Medici, D.; Hay, E.D.; Olsen, B.R. Snail and Slug promote epithelial-mesenchymal transition through β-catenin-T-cell factor-4-dependent expression of transforming growth factor-beta3. Mol. Biol. Cell 2008, 19, 4875–4887. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Zhang, G.; Zhang, H.; Zhang, F.; Zhou, B.; Ning, F.; Wang, H.S.; Cai, S.H.; Du, J. Acquisition of epithelial-mesenchymal transition phenotype and cancer stem cell-like properties in cisplatin-resistant lung cancer cells through AKT/β-catenin/Snail signaling pathway. Eur. J. Pharmacol. 2014, 723, 156–166. [Google Scholar] [CrossRef] [PubMed]
- Yook, J.I.; Li, X.Y.; Ota, I.; Fearon, E.R.; Weiss, S.J. Wnt-dependent regulation of the E-cadherin repressor snail. J. Biol. Chem. 2005, 280, 11740–11748. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Zhou, Q.; Liu, L.; Liu, P.; Pei, G.; Zeng, R.; Han, M.; Xu, G. Cdc42-Interacting Protein 4 Represses E-Cadherin Expression by Promoting β-Catenin Translocation to the Nucleus in Murine Renal Tubular Epithelial Cells. Int. J. Mol. Sci. 2015, 16, 19170-19183. https://doi.org/10.3390/ijms160819170
Xu C, Zhou Q, Liu L, Liu P, Pei G, Zeng R, Han M, Xu G. Cdc42-Interacting Protein 4 Represses E-Cadherin Expression by Promoting β-Catenin Translocation to the Nucleus in Murine Renal Tubular Epithelial Cells. International Journal of Molecular Sciences. 2015; 16(8):19170-19183. https://doi.org/10.3390/ijms160819170
Chicago/Turabian StyleXu, Chuou, Qiaodan Zhou, Lili Liu, Ping Liu, Guangchang Pei, Rui Zeng, Min Han, and Gang Xu. 2015. "Cdc42-Interacting Protein 4 Represses E-Cadherin Expression by Promoting β-Catenin Translocation to the Nucleus in Murine Renal Tubular Epithelial Cells" International Journal of Molecular Sciences 16, no. 8: 19170-19183. https://doi.org/10.3390/ijms160819170
APA StyleXu, C., Zhou, Q., Liu, L., Liu, P., Pei, G., Zeng, R., Han, M., & Xu, G. (2015). Cdc42-Interacting Protein 4 Represses E-Cadherin Expression by Promoting β-Catenin Translocation to the Nucleus in Murine Renal Tubular Epithelial Cells. International Journal of Molecular Sciences, 16(8), 19170-19183. https://doi.org/10.3390/ijms160819170




