GC–MS-Based Metabonomic Profiling Displayed Differing Effects of Borna Disease Virus Natural Strain Hu-H1 and Laboratory Strain V Infection in Rat Cortical Neurons
Abstract
:1. Introduction
2. Results
2.1. Taxonomical Classification of Borna Disease Virus (BDV) Strains Hu-H1 and Strain V
| Template | Hu-H1 (Abbreviation) | Strain V (Abbreviation) |
|---|---|---|
| Order | Mononegavirales | Mononegavirales |
| Family | Bornaviridae | Bornaviridae |
| Genus | Bornavirus | Bornavirus |
| Species | Borna disease virus (BDV) | BDV |
| Virus | BDV | BDV |
| Strain | BDV Hu-H1 (natural) | BDV Strain V (non-natural) |
| Isolation host | Homo sapiens (H. sapiens) | Equus ferus (E. ferus) |
| Isolation source | PBMCs | Brain |
| Sampling location | Germany (DEU), Berlin | Germany (DEU), Giessen |
| Sampling year | 1994 | 1927 |
| Genetic variant | Patient H1, female, 45 years., depressive episode, bipolar disorder (DSM IV 296.64), Berlin 1994, 3660-PBMC, passaged in human oligodendroglial (OL-221) cells (virus p11–p25), (stable virus titer from p11 on) | Horse V, fatal Borna disease (encephalomyelitis) (-hist), Giessen horse V brain, passaged in rabbits (brain, (20–50)×), rats (brain 6×), OL-221 cells (virus p10–p25) |
| Suffix | Passaged in OL cells (-tc) | Passaged in laboratory animal hosts and OL cells (-lab) |
| Pathology in rabbits | Behavioral disease (3 weeks p.i.), Hu-H1/OL p25 used | Fatal disease (3 weeks p.i.), Strain V/OL p12 used |
| GenBank accession No. | U58594, L76234, L76228, L76237 | U04608 (full-length) |
| Full designated name | Borna disease virus Hu-H1/H. sapiens-tc/DEU/1994/Berlin-Hu-H1-94 3660-PBMC | Borna disease virus Strain V/E. ferus VECTOR/O. cuniculus-lab/Wistar rat-lab/DEU/1927/Giessen-horse V-brain |
| Medium-length designation | BDV Hu-H1/Hsap-tc/DEU/94/Ber-Hu-H1-94 3660-PBMC | BDV Str. V/VECTOR/O. cun-lab/Wistar-lab/DEU/27/Gie-horse V-brain |
| Passage history of virus stock provided | OL-221/Hu-H1-94 3660, passage p75, 1994, provided 2010 to CQMU | OL-221/Strain V, passage p113, 1998, provided 2010 to CQMU |
| Passage grown at CQMU and used as virus source | OL-221/Hu-H1-94 3660, passage 77 in OL-221 passage 112 | OL-221/Strain V, passage 115 in OL-221 passage 116 |
2.2. Immunofluorescence Assay

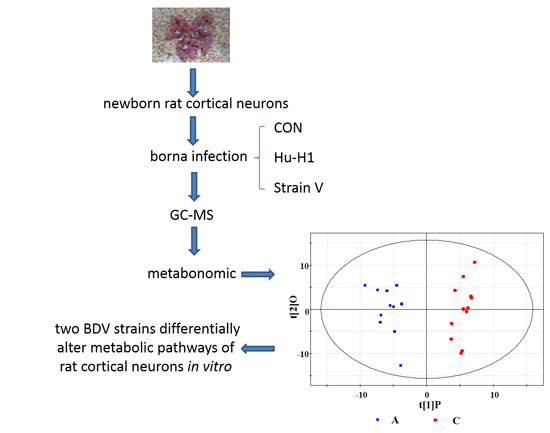
2.3. Metabonomic Analysis


| Hu-H1/Con | Metabolite | RT (min) | MZ | VIP-Value | p-Value (t-test) | Fold Change * |
| (OPLS-DA) | ||||||
| 1 | Citric acid | 14.63 | 273 | 2.42 | 1.1 × 10−10 | −1.49 |
| 2 | l-CysteineL | 11.61 | 218 | 1.86 | 0.000129 | −1.34 |
| 3 | Pyruvic acid | 5.05 | 174 | 2.19 | 4.58 × 10−7 | −1.29 |
| 4 | l-Aspartic acid | 11.2 | 233 | 2.14 | 0.00000144 | −1.18 |
| 5 | Desmosterol | 25.47 | 343 | 1.94 | 0.0000461 | −1.16 |
| 6 | Margaric acid | 17.6 | 117 | 2.35 | 3.73 × 10−9 | −1.03 |
| 7 | Glycerol-3-phosphate | 14.08 | 357 | 1.61 | 0.00167 | −0.8 |
| 8 | Sorbitol | 15.95 | 319 | 1.23 | 0.022 | −0.77 |
| 9 | l-Alanine | 5.69 | 116 | 1.95 | 0.0000402 | −0.67 |
| 10 | Myo-Inositol | 17.38 | 305 | 1.87 | 0.000112 | −0.66 |
| 11 | Serine | 9.18 | 204 | 2.03 | 0.0000118 | −0.58 |
| 12 | Phenylalanine | 12.43 | 218 | 1.16 | 0.0334 | −0.56 |
| 13 | l-Threonine | 9.54 | 218 | 1.84 | 0.000159 | −0.53 |
| 14 | Pyroglutamic acid | 11.19 | 156 | 1.27 | 0.018 | −0.49 |
| 15 | Malic acid | 10.82 | 233 | 1.31 | 1.46 × 10−2 | −0.47 |
| 16 | l-Methionine | 11.15 | 176 | 1.23 | 2.31 × 10−2 | −0.46 |
| 17 | l-Proline | 8.29 | 142 | 1.15 | 3.45 × 10−2 | −0.31 |
| 18 | Octadecanoic acid | 18.41 | 117 | 1.03 | 3.77 × 10−3 | −0.29 |
| 19 | l-Valine | 7.21 | 144 | 1.41 | 7.71 × 10−3 | −0.24 |
| 20 | l-Leucine | 7.97 | 158 | 1.24 | 2.22 × 10−2 | −0.21 |
| 21 | l-Isoleucine | 8.27 | 158 | 1.16 | 3.36 × 10−2 | −0.2 |
| 22 | Eicosanoic acid | 20.1 | 117 | 1.17 | 3.19 × 10−2 | 0.27 |
| 23 | d-Lactose | 22.47 | 204 | 1.17 | 3.16 × 10−2 | 0.31 |
| 24 | d-Glucose-6-phosphate | 19.65 | 315 | 1.15 | 3.49 × 10−2 | 0.49 |
| 25 | d-Fructose-6-phosphate | 19.4 | 315 | 1.25 | 1.99 × 10−2 | 0.54 |
| 26 | d-Fructose | 15.28 | 103 | 1.4 | 7.96 × 10−3 | 0.54 |
| 27 | Ethanolamine | 7.9 | 174 | 1.35 | 1.13 × 10−2 | 0.77 |
| 28 | 3-Hydroxy-3-methylglutaric acid | 12.16 | 247 | 1.86 | 1.23 × 10−4 | 0.94 |
| 29 | d-Glucose | 15.43 | 205 | 1.68 | 8.27 × 10−4 | 0.95 |
| 30 | Lanosterol | 25.35 | 458 | 1.9 | 7.37 × 10−5 | 0.96 |
| 31 | Methylsuccinic acid | 8.69 | 261 | 1.86 | 1.42 × 10−4 | 1.79 |
| Strain V/Con | Metabolite | RT (min) | MZ | VIP-Value | p-Value (t-test) | Fold Change * |
| (OPLS-DA) | ||||||
| 1 | d-Glucose-6-phosphate | 19.5 | 387 | 1.61 | 7.05 × 10−12 | −3.59 |
| 2 | d-Fructose-6-phosphate | 19.4 | 315 | 1.59 | 3.26 × 10−11 | −3.53 |
| 3 | l-Cysteine | 11.61 | 218 | 1.44 | 1.89 × 10−7 | −3.31 |
| 4 | γ-Aminobutyric acid | 11.27 | 174 | 1.57 | 2.04 × 10−10 | −3.22 |
| 5 | Citric acid | 14.63 | 273 | 1.66 | 4.82 × 10−16 | −3.22 |
| 6 | l-Lysine | 15.6 | 317 | 1.45 | 1.34 × 10−7 | −2.99 |
| 7 | Desmosterol | 25.47 | 343 | 1.5 | 1.60 × 10−8 | −2.84 |
| 8 | Pyruvic acid | 5.05 | 174 | 1.09 | 4.94 × 10−5 | −2.4 |
| 9 | Phenylalanine | 12.43 | 218 | 1.38 | 1.82 × 10−6 | −2.37 |
| 10 | Creatinine | 11.6 | 115 | 1.47 | 6.99 × 10−8 | −2.27 |
| 11 | l-Aspartic acid | 11.2 | 232 | 1.52 | 1.61 × 10−8 | −2.06 |
| 12 | l-Methionine | 11.15 | 176 | 1.42 | 3.76 × 10−7 | −1.97 |
| 13 | Serine | 9.18 | 204 | 1.59 | 4.36 × 10−11 | −1.7 |
| 14 | Malic acid | 10.82 | 233 | 1.38 | 1.44 × 10−6 | −1.64 |
| 15 | l-Alanine | 5.69 | 116 | 1.51 | 1.14 × 10−8 | −1.56 |
| 16 | d-Fructose | 15.28 | 103 | 1.6 | 8.56 × 10−12 | −1.51 |
| 17 | l-Glutamic acid | 12.36 | 246 | 1.17 | 2.07 × 10−4 | −1.51 |
| 18 | l-Threonine | 9.54 | 218 | 1.55 | 6.11 × 10−10 | −1.41 |
| 19 | d-Glucose | 15.43 | 205 | 1.46 | 1.15 × 10−7 | −1.4 |
| 20 | Glycerol-3-phosphate | 14.08 | 357 | 1.27 | 3.36 × 10−5 | −1.28 |
| 21 | l-Proline | 8.29 | 142 | 1.4 | 9.06 × 10−7 | −1.22 |
| 22 | Myo-Inositol | 17.38 | 305 | 1.41 | 6.71 × 10−7 | −1.2 |
| 23 | Ribulose-5-phosphate | 17.72 | 357 | 1.24 | 5.19 × 10−5 | −0.99 |
| 24 | Margaric acid | 17.6 | 117 | 1.42 | 4.04 × 10−7 | −0.97 |
| 25 | Myo-Inositol-1-phosphate | 20.26 | 318 | 1.19 | 1.47 × 10−4 | −0.96 |
| 26 | l-Valine | 7.21 | 144 | 1.51 | 7.91 × 10−9 | −0.95 |
| 27 | l-Leucine | 7.97 | 158 | 1.46 | 9.99 × 10−8 | −0.91 |
| 28 | Pyroglutamic acid | 11.19 | 156 | 1.15 | 3.22 × 10−4 | −0.91 |
| 29 | l-Isoleucine | 8.27 | 158 | 1.45 | 1.34 × 10−7 | −0.78 |
| 30 | Oleic acid | 18.2 | 117 | 1.15 | 3.09 × 10−3 | −0.55 |
| 31 | Eicosanoic acid | 20.1 | 117 | 1.02 | 1.95 × 10−3 | 0.45 |
| 32 | Glycerol | 8.05 | 205 | 1.11 | 5.74 × 10−4 | 0.56 |
| 33 | Glycolic acid | 5.35 | 177 | 1.11 | 5.85 × 10−4 | 0.57 |
| 34 | Ethanolamine | 7.9 | 174 | 1 | 2.52 × 10−3 | 0.77 |
| 35 | Methylsuccinic acid | 8.69 | 261 | 1.25 | 5.18 × 10−5 | 1.64 |
| Hu-H1/Strain V | Metabolite | RT (min) | MZ | VIP-Value (OPLS-DA) | p-Value (t-test) Hu-H1 Strain V | Fold Change Hu-H1/CON Strain V/CON | |||
|---|---|---|---|---|---|---|---|---|---|
| Hu-H1 | Strain V | ||||||||
| 1 | Citric acid | 14.63 | 273 | 2.42 | 1.66 | 1.10 × 10−10 | 4.82 × 10−16 | −1.49 | −3.22 |
| 2 | l-CysteineL | 11.61 | 218 | 1.86 | 1.44 | 0.000129 | 1.89 × 10−7 | −1.34 | −3.31 |
| 3 | Pyruvic acid | 5.05 | 174 | 2.19 | 1.09 | 4.58 × 10−7 | 4.94 × 10−5 | −1.29 | −2.4 |
| 4 | l-Aspartic acid | 11.2 | 233 | 2.14 | 1.52 | 0.00000144 | 1.61 × 10−8 | −1.18 | −2.06 |
| 5 | Desmosterol | 25.47 | 343 | 1.94 | 1.5 | 0.0000461 | 1.60 × 10−8 | −1.16 | −2.84 |
| 6 | Margaric acid | 17.6 | 117 | 2.35 | 1.42 | 3.73 × 10−9 | 4.04 × 10−7 | −1.03 | −0.97 |
| 7 | Glycerol-3-phosphate | 14.08 | 357 | 1.61 | 1.27 | 0.00167 | 3.36 × 10−5 | −0.8 | −1.28 |
| 8 | l-Alanine | 5.69 | 116 | 1.95 | 1.51 | 0.0000402 | 1.14 × 10−8 | −0.67 | −1.56 |
| 9 | Myo-Inositol | 17.38 | 305 | 1.87 | 1.41 | 0.000112 | 6.71 × 10−7 | −0.66 | −1.2 |
| 10 | Serine | 9.18 | 204 | 2.03 | 1.59 | 0.0000118 | 4.36 × 10−11 | −0.58 | −1.7 |
| 11 | Phenylalanine | 12.43 | 218 | 1.16 | 1.38 | 0.0334 | 1.82 × 10−6 | −0.56 | −2.37 |
| 12 | l-Threonine | 9.54 | 218 | 1.84 | 1.55 | 0.000159 | 6.11 × 10−10 | −0.53 | −1.41 |
| 13 | Pyroglutamic acid | 11.19 | 156 | 1.27 | 1.15 | 0.018 | 3.22 × 10−4 | −0.49 | −0.91 |
| 14 | Malic acid | 10.82 | 233 | 1.31 | 1.38 | 1.46 × 10−2 | 1.44 × 10−6 | −0.47 | −1.64 |
| 15 | l-Methionine | 11.15 | 176 | 1.23 | 1.42 | 2.31 × 10−2 | 3.76 × 10−7 | −0.46 | −1.97 |
| 16 | l-Proline | 8.29 | 142 | 1.15 | 1.4 | 3.45 × 10−2 | 9.06 × 10−7 | −0.31 | −1.22 |
| 17 | l-Valine | 7.21 | 144 | 1.41 | 1.51 | 7.71 × 10−3 | 7.91 × 10−9 | −0.24 | −0.95 |
| 18 | l-Leucine | 7.97 | 158 | 1.24 | 1.46 | 2.22 × 10−2 | 9.99 × 10−8 | −0.21 | −0.91 |
| 19 | l-Isoleucine | 8.27 | 158 | 1.16 | 1.45 | 3.36 × 10−2 | 1.34 × 10−7 | −0.2 | −0.78 |
| 20 | Eicosanoic acid | 20.1 | 117 | 1.17 | 1.02 | 3.19 × 10−2 | 1.95 × 10−3 | 0.27 | 0.45 |
| 21 | Ethanolamine | 7.9 | 174 | 1.35 | 1 | 1.13 × 10−2 | 2.52 × 10−3 | 0.77 | 0.77 |
| 22 | Methylsuccinic acid | 8.69 | 261 | 1.86 | 1.25 | 1.42 × 10−4 | 0.0000518 | 1.79 | 1.64 |
| Hu-H1/Strain V | Metabolite | RT (min) | MZ | VIP-Value | p-Value (t-test) | Fold Change * |
|---|---|---|---|---|---|---|
| (OPLS-DA) | ||||||
| 1 | Glycolic acid | 5.35 | 177 | 1.36 | 3.71 × 10−5 | −0.75 |
| 2 | Myo-Inositol-1-phosphate | 20.26 | 318 | 1.06 | 8.76 × 10−4 | −0.68 |
| 3 | d-Fructose-6-phosphate | 19.4 | 315 | 1.6 | 4.89 × 10−5 | −0.62 |
| 4 | Glycerol | 8.05 | 205 | 1.11 | 1.86 × 10−5 | −0.37 |
| 5 | Cholesterol | 25.21 | 129 | 1.05 | 2.42 × 10−2 | −0.36 |
| 6 | Desmosterol | 25.47 | 343 | 1.6 | 3.96 × 10−2 | −0.34 |
| 7 | Lanosterol | 25.35 | 458 | 1.26 | 7.60 × 10−2 | −0.25 |
| 8 | Citric acid | 14.63 | 273 | 1.53 | 1.62 × 10−1 | −0.2 |
| 9 | d-Glucose-6-phosphate | 19.5 | 387 | 1.59 | 7.22 × 10−2 | 0.39 |
| 10 | Myo-Inositol | 17.38 | 305 | 1.09 | 4.37 × 10−2 | 0.48 |
| 11 | l-Isoleucine | 8.27 | 158 | 1.41 | 1.43 × 10−5 | 0.58 |
| 12 | Ribulose-5-phosphate | 17.72 | 357 | 1.2 | 1.52 × 10−3 | 0.61 |
| 13 | l-Leucine | 7.97 | 158 | 1.41 | 1.25 × 10−5 | 0.7 |
| 14 | l-Valine | 7.21 | 144 | 1.53 | 5.61 × 10−7 | 0.71 |
| 15 | Glycine | 8.43 | 174 | 1.5 | 1.58 × 10−6 | 0.83 |
| 16 | d-Fructose | 15.28 | 103 | 1.55 | 1.08 × 10−3 | 0.88 |
| 17 | l-Threonine | 9.54 | 218 | 1.39 | 2.11 × 10−5 | 0.89 |
| 18 | l-Alanine | 5.69 | 116 | 1.43 | 8.28 × 10−6 | 0.89 |
| 19 | l-Proline | 8.29 | 142 | 1.37 | 3.67 × 10−5 | 0.92 |
| 20 | Serine | 9.18 | 204 | 1.49 | 1.66 × 10−6 | 1.13 |
| 21 | d-Glucose | 15.43 | 205 | 1.51 | 3.58 × 10−8 | 1.14 |
3. Discussion
3.1. Energy Metabolism
3.2. Cholesterol and Fatty Acid Metabolism
3.3. Amino Acid Metabolism

4. Experimental Section
4.1. History and Proposed Taxonomy of Virus Strains
4.2. Primary Culture of Neurons and Viral Infections
4.3. Immunofluorescence
4.4. Metabolite Extraction
4.5. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.6. Metabonomic Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Kinnunen, P.M.; Palva, A.; Vaheri, A.; Vapalahti, O. Epidemiology and host spectrum of Borna disease virus infections. J. Gen. Virol. 2013, 94 Pt 2, 247–262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, X.; Zhan, Q.; Wang, Z.; Xu, M.; Zhu, D.; He, F.; Liu, X.; Huang, R.; Li, D.; et al. Evidence for natural Borna disease virus infection in healthy domestic animals in three areas of western China. Arch. Virol. 2014, 159, 1941–1949. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Xu, M.M.; Zeng, L.; Liu, S.; Liu, X.; Wang, X.; Li, D.; Huang, R.Z.; Zhao, L.B.; Zhan, Q.L.; et al. Evidence for Borna disease virus infection in neuropsychiatric patients in three western China provinces. Eur. J. Clin. Microbiol. Infect. Dis. 2014, 33, 621–627. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Bode, L.; Gosztonyi, G. Borna disease: A persistent virus infection of the central nervous system. Prog. Med. Virol. 1988, 35, 107–151. [Google Scholar] [PubMed]
- Bode, L.; Dürrwald, R.; Ludwig, H. Borna virus infections in cattle associated with fatal neurological disease. Vet. Rec. 1994, 135, 283–284. [Google Scholar] [CrossRef] [PubMed]
- Lundgren, A.L.; Lindberg, R.; Ludwig, H.; Gosztonyi, G. Immunoreactivity of the central nervous system in cats with a Borna disease-like meningoencephalomyelitis (staggering disease). Acta Neuropathol. 1995, 90, 184–193. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Tsuge, Y.; Asakawa, M.; Kabaya, H.; Okamoto, M.; Miyasho, T.; Taniyama, H.; Ishihara, C.; de la Torre, J.C.; Ikuta, K. Borna disease virus RNA detected in Japanese macaques (Macaca fuscata). Primates 2008, 49, 57–64. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Ludwig, H. Borna disease virus infection, a human mental-health risk. Clin. Microbiol. Rev. 2003, 16, 534–545. [Google Scholar] [CrossRef] [PubMed]
- Ikuta, K.; Ibrahim, M.S.; Kobayashi, T.; Tomonaga, K. Borna disease virus and infection in humans. Front. Biosci. 2002, 7, d470–d495. [Google Scholar] [CrossRef] [PubMed]
- Kistler, A.L.; Gancz, A.; Clubb, S.; Skewes-Cox, P.; Fischer, K.; Sorber, K.; Chiu, C.Y.; Lublin, A.; Mechani, S.; Farnoushi, Y.; et al. Recovery of divergent avian bornaviruses from cases of proventricular dilatation disease: Identification of a candidate etiologic agent. Virol. J. 2008, 5, 88. [Google Scholar] [CrossRef] [PubMed]
- Horie, M.; Honda, T.; Suzuki, Y.; Kobayashi, Y.; Daito, T.; Oshida, T.; Ikuta, K.; Jern, P.; Gojobori, T.; Coffin, J.M.; et al. Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature 2010, 463, 84–87. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Bode, L. Borna disease virus: New aspects on infection, disease, diagnosis and epidemiology. Rev. Sci. Tech. 2000, 19, 259–288. [Google Scholar] [PubMed]
- Pletnikov, M.V.; Rubin, S.A.; Schwartz, G.J.; Carbone, K.M.; Moran, T.H. Effects of neonatal rat Borna disease virus (BDV) infection on the postnatal development of the brain monoaminergic systems. Brain Res. Dev. Brain Res. 2000, 119, 179–185. [Google Scholar] [CrossRef]
- Gosztonyi, G. Natural and experimental Borna disease virus infections—Neuropathology and pathogenetic considerations. APMIS Suppl. 2008, 116, 53–57. [Google Scholar] [CrossRef]
- Briese, T.; Schneemann, A.; Lewis, A.J.; Park, Y.S.; Kim, S.; Ludwig, H.; Lipkin, W.I. Genomic organization of Borna disease virus. Proc. Natl. Acad. Sci. USA 1994, 91, 4362–4366. [Google Scholar] [CrossRef] [PubMed]
- Pringle, C.R. Virus taxonomy 1996—A bulletin from the Xth International Congress of Virology in Jerusalem. Arch. Virol. 1996, 141, 2251–2256. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Dürrwald, R.; Bào, Y.; Briese, T.; Carbone, K.; Clawson, A.N.; deRisi, J.L.; Garten, W.; Jahrling, P.B.; Kolodziejek, J.; et al. Taxonomic reorganization of the family Bornaviridae. Arch. Virol. 2015, 160, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Bode, L.; Durrwald, R.; Rantam, F.A.; Ferszt, R.; Ludwig, H. First isolates of infectious human Borna disease virus from patients with mood disorders. Mol. Psychiatry 1996, 1, 200–212. [Google Scholar] [PubMed]
- Zwick, W. Neuere Untersuchungen über die seuchenhafte Gehirn-u. Rückenmarksentzündung (Borna’sche Krankheit) der Pferde. Dtsch. Z. Nervenheilk. 1929, 110, 316–322. [Google Scholar] [CrossRef]
- Hirano, N.; Kao, M.; Ludwig, H. Persistent, tolerant or subacute infection in Borna disease virus-infected rats. J. Gen. Virol. 1983, 64, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Bao, Y.; Bavari, S.; Becker, S.; Bradfute, S.; Brister, J.R.; Bukreyev, A.A.; Chandran, K.; Davey, R.A.; Dolnik, O.; et al. Virus nomenclature below the species level: A standardized nomenclature for natural variants of viruses assigned to the family Filoviridae. Arch. Virol. 2013, 158, 301–311. [Google Scholar] [CrossRef] [PubMed]
- Kuhn, J.H.; Bao, Y.; Bavari, S.; Becker, S.; Bradfute, S.; Brister, J.R.; Bukreyev, A.A.; Caì, Y.; Chandran, K.; Davey, R.A.; et al. Virus nomenclature below the species level: A standardized nomenclature for laboratory animal-adapted strains and variants of viruses assigned to the family Filoviridae. Arch. Virol. 2013, 158, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Huang, R.; Gao, H.; Zhang, L.; Jia, J.; Liu, X.; Zheng, P.; Ma, L.; Li, W.; Deng, J.; Wang, X.; et al. Borna disease virus infection perturbs energy metabolites and amino acids in cultured human oligodendroglia cells. PLoS ONE 2012, 7, e44665. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Yang, Y.; Zhao, M.; Bode, L.; Zhang, L.; Pan, J.; Lv, L.; Zhan, Y.; Liu, S.; Zhang, L.; et al. Proteomics reveal energy metabolism and mitogen-activated protein kinase signal transduction perturbation in human Borna disease virus Hu-H1-infected oligodendroglial cells. Neuroscience 2014, 268, 284–296. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Liu, S.; Bode, L.; Zhang, L.; Wang, X.; Liu, C.; Li, D.; Lei, Y.; Peng, X.; Cheng, Z.; et al. Persistent human Borna disease virus infection modifies the acetylome of human oligodendroglia cells towards higher energy and transporter levels. Virology 2015, 485, 58–78. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Lei, Y.; Deng, J.; Zhou, C.; Zhang, Y.; Li, W.; Huang, H.; Cheng, S.; Zhang, H.; Zhang, L.; et al. Human but not laboratory Borna disease virus inhibits proliferation and induces apoptosis in human oligodendrocytes in vitro. PLoS ONE 2013, 8, e66623. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.; Gao, H.C.; Li, Q.; Shao, W.H.; Zhang, M.L.; Cheng, K.; Yang, D.Y.; Fan, S.H.; Chen, L.; Fang, L.; et al. Plasma metabonomics as a novel diagnostic approach for major depressive disorder. J. Proteome Res. 2012, 11, 1741–1748. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.; Bajad, S.U.; Coller, H.A.; Shenk, T.; Rabinowitz, J.D. Dynamics of the cellular metabolome during human cytomegalovirus infection. PLoS Pathog. 2006, 2, e132. [Google Scholar] [CrossRef] [PubMed]
- Akhtar, S.N.; Singh, R.K.; Jadegoud, Y.; Dhole, T.N.; Ayyagari, A.; Nagana Gowda, G.A. In vitro 1H NMR studies of RD human cell infection with echovirus 11. NMR Biomed. 2007, 20, 422–428. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Tang, G.; Cheng, K.; Yang, D.; Chen, G.; Liu, Z.; Zhang, R.; Zhou, J.; Fang, L.; Fang, Z.; et al. Peripheral blood mononuclear cell-based metabolomic profiling of a chronic unpredictable mild stress rat model of depression. Mol. Biosyst. 2014, 10, 2994–3001. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, E.L.; Lagunoff, M. Viral activation of cellular metabolism. Virology 2015, 479–480, 609–618. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Li, D.; Deng, J.; Shao, W.; Fan, S.; Wang, X.; Huang, H.; Chen, S.; Zhang, H.; Zhang, L.; et al. Metabolomic profiling of three brain regions from a postnatal infected Borna disease virus Hu-H1 rat model. Metabolomics 2014, 10, 484–495. [Google Scholar] [CrossRef]
- Zhang, L.; Lei, Y.; Liu, X.; Wang, X.; Liu, Z.; Li, D.; Zheng, P.; Zhang, L.; Chen, S.; Xie, P. Glutamate and lipid metabolic perturbation in the hippocampi of asymptomatic Borna disease virus-infected horses. PLoS ONE 2014, 23, e99752. [Google Scholar] [CrossRef] [PubMed]
- De la Torre, J.C.; Bode, L.; Durrwald, R.; Cubitt, B.; Ludwig, H. Sequence characterization of human Borna disease virus. Virus Res. 1996, 44, 33–44. [Google Scholar] [CrossRef]
- Van Regenmortel, M.H. Virus species and virus identification: Past and current controversies. Infect. Genet. Evol. 2007, 7, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Liu, S.; Zhang, L.; You, H.; Huang, R.; Sun, L.; He, P.; Chen, S.; Zhang, H.; Xie, P. Real-time qPCR identifies suitable reference genes for Borna disease virus-infected rat cortical neurons. Int. J. Mol. Sci. 2014, 15, 21825–21839. [Google Scholar] [CrossRef] [PubMed]
- Hornig, M.; Solbrig, M.; Horscroft, N.; Weissenbock, H.; Lipkin, W.I. Borna disease virus infection of adult and neonatal rats: Models for neuropsychiatric disease. Curr. Top. Microbiol. Immunol. 2001, 253, 157–177. [Google Scholar] [PubMed]
- Dürrwald, R.; Kolodziejek, J.; Herzog, S.; Nowotny, N. Meta-analysis of putative human bornavirus sequences fails to provide evidence implicating Borna disease virus in mental illness. Rev. Med. Virol. 2007, 17, 181–203. [Google Scholar] [CrossRef] [PubMed]
- Ritter, J.B.; Wahl, A.S.; Freund, S.; Genzel, Y.; Reichl, U. Metabolic effects of influenza virus infection in cultured animal cells: Intra- and extracellular metabolite profiling. BMC Syst. Biol. 2010, 4, 61. [Google Scholar] [CrossRef] [PubMed]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef] [PubMed]
- Vastag, L.; Koyuncu, E.; Grady, S.L.; Shenk, T.E.; Rabinowitz, J.D. Divergent effects of human cytomegalovirus and herpes simplex virus-1 on cellular metabolism. PLoS Pathog. 2011, 7, e1002124. [Google Scholar] [CrossRef] [PubMed]
- Predmore, Z.S. Exploring the Effects of Human Cytomegalovirus Infection on Host Cell Metabolism: A Proteomic Approach. Ph.D. Thesis, Princeton University, Princeton, NJ, USA, 2011. [Google Scholar]
- Jiang, P.; Du, W.; Wu, M. Regulation of the pentose phosphate pathway in cancer. Protein Cell 2014, 5, 592–602. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Liu, N.; Yang, Z.; Song, W.; Wang, P.; Chen, H.; Lucio, M.; Schmitt-Kopplin, P.; Chen, G.; Cai, Z. GC/MS-based metabolomics reveals fatty acid biosynthesis and cholesterol metabolism in cell lines infected with influenza A virus. Talanta 2010, 83, 262–268. [Google Scholar] [CrossRef] [PubMed]
- Lou, H.C. Dopamine precursors and brain function in phenylalanine hydroxylase deficiency. Acta Paediatr. Suppl. 1994, 407, 86–88. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.X.; Ofiyai, H.; He, M.; Bu, X.; Wen, Y.; Jia, W. Neuronal activity regulates viral replication of herpes simplex virus type 1 in the nervous system. J. Neurovirol. 2005, 11, 256–624. [Google Scholar] [CrossRef] [PubMed]
- Moss, S.J.; Smart, T.G. Constructing inhibitory synapses. Nat. Rev. Neurosci. 2001, 2, 240–250. [Google Scholar] [CrossRef] [PubMed]
- Peng, G.; Yan, Y.; Zhu, C.; Wang, S.; Yan, X.; Lu, L.; Li, W.; Hu, J.; Wei, W.; Mu, Y.; et al. Borna disease virus P protein affects neural transmission through interactions with γ-aminobutyric acid receptor-associated protein. J. Virol. 2008, 82, 12487–12497. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.E.; Geballe, M.T.; Stansfeld, P.J.; Johnston, A.R.; Yuan, H.; Jacob, A.L.; Snyder, J.P.; Traynelis, S.F.; Wyllie, D.J. Structural features of the glutamate binding site in recombinant NR1/NR2A N-methyl-d-aspartate receptors determined by site-directed mutagenesis and molecular modeling. Mol. Pharmacol. 2005, 67, 1470–1484. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.H.; Han, T.Z. Glycine modulates synaptic NR2A- and NR2B-containing NMDA receptor-mediated responses in the rat visual cortex. Brain Res. 2008, 1190, 49–55. [Google Scholar] [CrossRef] [PubMed]
- Fleck, M.W.; Henze, D.A.; Barrionuevo, G.; Palmer, A.M. Aspartate and glutamate mediate excitatory synaptic transmission in area CA1 of the hippocampus. J. Neurosci. 1993, 13, 3944–3955. [Google Scholar] [PubMed]
- Ovanesov, M.V.; Vogel, M.W.; Moran, T.H.; Pletnikov, M.V. Neonatal Borna disease virus infection in rats is associated with increased extracellular levels of glutamate and neurodegeneration in the striatum. J. Neurovirol. 2007, 13, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Billaud, J.N.; Ly, C.; Phillips, T.R.; de la Torre, J.C. Borna disease virus persistence causes inhibition of glutamate uptake by feline primary cortical astrocytes. J. Virol. 2000, 74, 10438–10446. [Google Scholar] [CrossRef] [PubMed]
- Bajramovic, J.J.; Munter, S.; Syan, S.; Nehrbass, U.; Brahic, M.; Gonzalez-Dunia, D. Borna disease virus glycoprotein is required for viral dissemination in neurons. J. Virol. 2003, 77, 12222–12231. [Google Scholar] [CrossRef] [PubMed]
- Suberbielle, E.; Stella, A.; Pont, F.; Monnet, C.; Mouton, E.; Lamouroux, L.; Monsarrat, B.; Gonzalez-Dunia, D. Proteomic analysis reveals selective impediment of neuronal remodeling upon Borna disease virus infection. J. Virol. 2008, 82, 12265–12279. [Google Scholar] [CrossRef] [PubMed]
- Ludwig, H.; Furuya, K.; Bode, L.; Klein, N.; Durrwald, R.; Lee, D.S. Biology and neurobiology of Borna disease viruses (BDV), defined by antibodies, neutralizability and their pathogenic potential. Arch. Virol. Suppl. 1993, 7, 111–133. [Google Scholar] [PubMed]
- Luedemann, A.; Strassburg, K.; Erban, A.; Kopka, J. TagFinder for the quantitative analysis of gas chromatography–mass spectrometry (GC–MS)-based metabolite profiling experiments. Bioinformatics (Oxf. Engl.) 2008, 24, 732–737. [Google Scholar] [CrossRef] [PubMed]
- Aarts, K.; Vanderhasselt, M.A.; Otte, G.; Baeken, C.; Pourtois, G. Electrical brain imaging reveals the expression and timing of altered error monitoring functions in major depression. J. Abnorm. Psychol. 2013, 122, 939–950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Bode, L.; Zhang, L.; He, P.; Huang, R.; Sun, L.; Chen, S.; Zhang, H.; Guo, Y.; Zhou, J.; et al. GC–MS-Based Metabonomic Profiling Displayed Differing Effects of Borna Disease Virus Natural Strain Hu-H1 and Laboratory Strain V Infection in Rat Cortical Neurons. Int. J. Mol. Sci. 2015, 16, 19347-19368. https://doi.org/10.3390/ijms160819347
Liu S, Bode L, Zhang L, He P, Huang R, Sun L, Chen S, Zhang H, Guo Y, Zhou J, et al. GC–MS-Based Metabonomic Profiling Displayed Differing Effects of Borna Disease Virus Natural Strain Hu-H1 and Laboratory Strain V Infection in Rat Cortical Neurons. International Journal of Molecular Sciences. 2015; 16(8):19347-19368. https://doi.org/10.3390/ijms160819347
Chicago/Turabian StyleLiu, Siwen, Liv Bode, Lujun Zhang, Peng He, Rongzhong Huang, Lin Sun, Shigang Chen, Hong Zhang, Yujie Guo, Jingjing Zhou, and et al. 2015. "GC–MS-Based Metabonomic Profiling Displayed Differing Effects of Borna Disease Virus Natural Strain Hu-H1 and Laboratory Strain V Infection in Rat Cortical Neurons" International Journal of Molecular Sciences 16, no. 8: 19347-19368. https://doi.org/10.3390/ijms160819347