Size Does Matter: Staging of Silene latifolia Floral Buds for Transcriptome Studies
Abstract
:1. Introduction
2. Results and Discussion
2.1. Infection Method
2.2. Floral Size and Staging

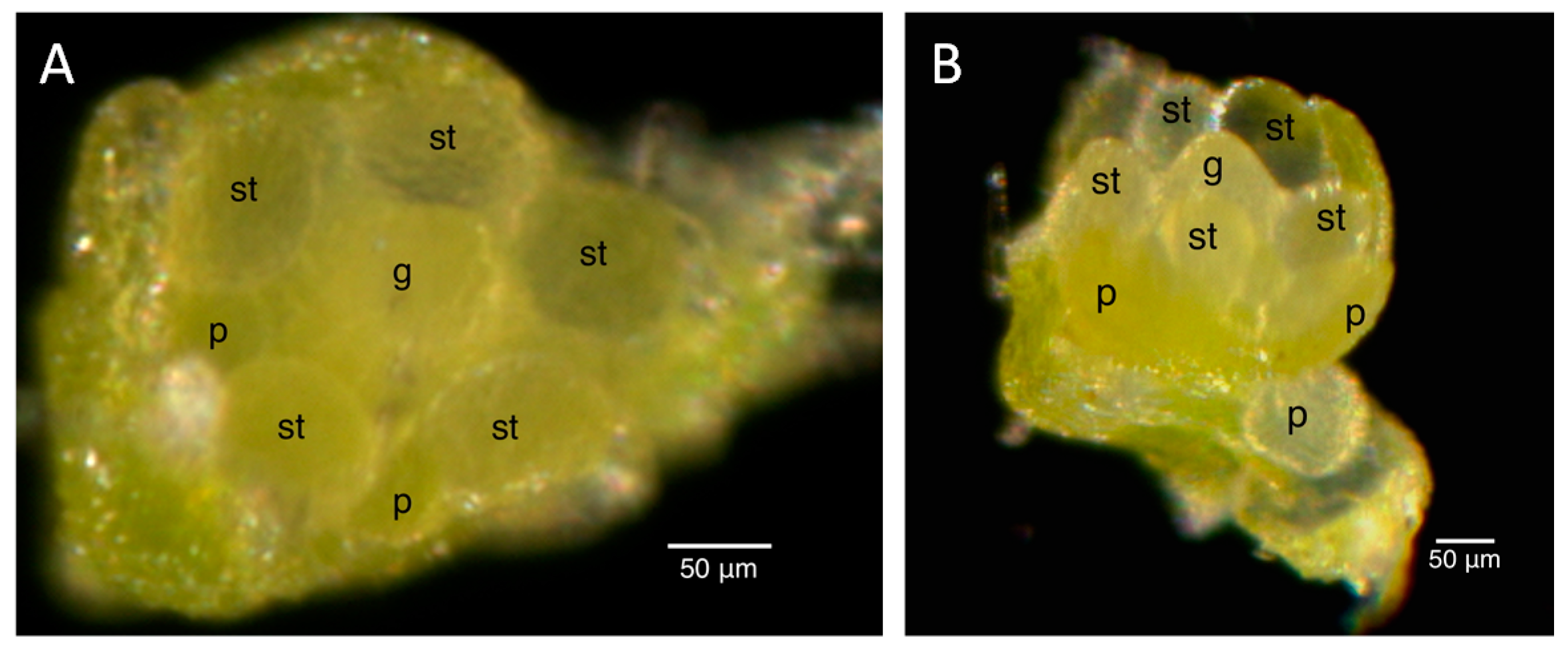




| Bud Size (Rough) | Bud Size (Scope) | Shape of Gynoecium a | External Anthers | Internal Anthers | Petals | Stage |
|---|---|---|---|---|---|---|
| <1 (in sepal of 1.5 mm) | - | Petal and stamen primordia start to develop | 5 | |||
| <1 (in sepal of 2 mm) | - | “mushroom” shaped with petal primordia separated from internal stamen primordia | - | - | Not visible | 6 |
| <1 | 0.8 | - | Unilobal | - | Not visible | 7 |
| <1 | 0.7 | Shallow “barrel and star” | 0.1 mm bilobal | 0.05 mm unilobal | Not visible | 7 |
| 0.5 | 0.7 | “barrel and star” shape | 0.075 mm bilobal | 0.05 mm unilobal | Not visible | 7 |
| 1 | 1.4 | elongated “barrel and star” | 0.25 mm tetralobal | 0.15 mm bilobal | Not observed | 8 |
| 1 | 1.5 | elongated “barrel and star” | 0.1 mm bilobal | Not observed | Not visible | 8 |
| 1.5 | 1.75 | elongated “barrel and star” | 0.45 mm tetralobal | 0.35 mm tetralobal | 0.2–0.3 mm cordiform | 8 |
| 2 | 1.75 | gynoecium beginning to close | 0.35 mm tetralobal | 0.25 mm tetralobal | small tongue-like | 9 |
| 2 | 2.25 | gynoecium beginning to close | 0.2 mm tetralobal | Not observed | tongue-like | 9 |
| 3 | 2.5 | gynoecium beginning to close | 0.45 mm tetralobal | 0.3 mm tetralobal | 0.4 mm tongue-like | 9 |
| 3 | 2.5 | Styles appearing | 0.2 mm tetralobal | Not observed | Overlapping | 10 |
| 3.5 | 3.25 | elongated gynoecium and closing of “barrel” | 0.65 mm tetralobal | 0.65 mm tetralobal | 1 mm slight cordiform | 9–10 |
| 3.5 | 3.25 | Styles appearing | 0.65 mm tetralobal | 0.55 mm tetralobal | 0.7 mm cordiform | 10 |
| 3 | 3 | Styles appearing | 0.7 mm tetralobal | 0.6 mm tetralobal | 0.7 mm cordiform | 10 |
| 4 | 3.5 | Style appearing | 0.2 mm tetralobal | Not observed | 0.75 mm | 10 |
| 4 | 4 | Visible styles | 0.75 mm tetralobal | 0.6 mm tetralobal | 1 mm cordiform | 10 |
| 5 | 5 | Elongation of styles | 0.35 mm tetralobal | Not observed | 1.25 mm | >11 |
| 5 | 5.5 | Short style and stunted gynoecium | 1.2 mm tetralobal | 0.8 mm tetralobal | 2.5 mm full petal | - |
| 7 | 7.5 | Elongated gynoecium with stunted style | 2 mm tetralobal | 1.25 mm tetralobal | 5.5 mm full petal | >11 |
| 8 | 8.5 | Long style | 0.75 mm tetralobal | 0.25 mm tetralobal | 4 mm full petal | >11 |
| Bud Size (Rough) | Bud Size (Scope) | Shape of Gynoecium a | Petals | Stage |
|---|---|---|---|---|
| <1 | 0.7 | “barrel and star” shape | Visible and not overlapping | 7 |
| <1 | 0.7 | “barrel and star” shape | Hardly visible | 7 |
| 1 | 1.1 | “barrel and star” shape | Visible and larger than others | 7 |
| 1 | 1.2 | elongated “barrel and star” | Tongue-like | 8 |
| Slightly > 1 mm | 1.5 | elongated “barrel and star” | 0.7 mm | 8 |
| 1 | 1.5 | elongated “barrel and star” | Cordiform | 8 |
| 1.5 | 1.5 | elongated “barrel and star” | Hardly distinguishable from anthers | 8 |
| 2 | 1.75 | elongated “barrel and star” | Visible | 8 |
| 2 | 2 | elongated “barrel and star” | Visible and not overlapping | 8 |
| 2 | 2 | elongated “barrel and star” | No petal | 8 |
| 2.5 | 2.5 | elongated “barrel and star” | 0.2 mm transparent cordiform | 8 |
| 2.5 | 2.75 | gynoecium beginning to close | 0.3 mm cordiform | 9 |
| 3 | 2.5 | Styles appearing | Visible and not overlapping | 10 |
| 3 | 3 | Visible styles | 0.35 mm cordiform | 10.1 |
| 4 | 4 | Styles appearing | Visible and overlapping | 10 |
| 4 | 4 | Visible styles | 0.75 mm cordiform | Mid 10 |
| 4 | 4 | Visible styles | 0.75 mm cordiform | 10.2 |
| 4 | 4.5 | Visible styles | 0.9 mm cordiform | 10 |
| 4.5 | 4.5 | Visible styles | 1.5 mm fully formed | 10 |
| 5 | 5 | Long styles, petal half of ovary | 1.5 mm half of ovary | 10 |
| 6 | 6 | Long styles, petal as long as ovary | 2.5 mm, covers ovary | >11 |
| 7 | 7 | Long styles, petal as long as ovary | 3.5 mm, covers ovary | >11 |
| 7 | 6 | Long styles, petal as long as ovary | 1.75 mm fully formed | >11 |



2.3. RNA Extraction
2.4. Unusual Floral Buds
| Tissue Type | Stage | Weight of Tissue (mg) | Amount of Crude RNA (μg) | RNA Integrity Number (RIN) a |
|---|---|---|---|---|
| Male infected | 8 | 91 | 64 | N/A |
| Male infected | 8 | 38.2 | 62 | 9.4 |
| Male infected | 9 | 150.6 | 61 | N/A |
| Male infected | 9 | 101.5 | 85 | 8.9 |
| Male infected | 10 | 116.2 | 180 | 9 |
| Male infected | 10 | 110.2 | 115 | 9 |
| Female infected | 7 | 34.7 | 51 | 6.4 |
| Female infected | 9 | 55.2 | 91 | 6.4 |
| Female infected | 10 | 187.8 | 143 | 9.3 |
| Male uninfected | 8 | 28 | 45 | 6.9 |
| Male uninfected | 9 | 34.4 | 40 | 6.1 |
| Male uninfected | 9 | 19.2 | 63 | N/A |
| Female uninfected | 9 | 30 | 104 | 6.3 |
| Female uninfected | 10 | 90 | 103 | 6.2 |
| Female uninfected | 10 | 109 | 120 | N/A |

3. Experimental Section
3.1. Germination
3.2. Infection with Microbotryum lychnidis-dioicae
3.3. Floral Bud Collection and Examination
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Filatov, D.A. Substitution rates in a new Silene latifolia sex-linked gene, SlssX/Y. Mol. Biol. Evol. 2005, 22, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Guttman, D.S.; Charlesworth, D. An X-linked gene with a degenerate Y-linked homologue in a dioecious plant. Nature 1998, 393, 263–266. [Google Scholar] [CrossRef] [PubMed]
- Nicolas, M.; Marais, G.; Hykelova, V.; Janousek, B.; Laporte, V.; Vyskot, B.; Mouchiroud, D.; Negrutiu, I.; Charlesworth, D.; Monéger, F. A gradual process of recombination restriction in the evolutionary history of the sex chromosomes in dioecious plants. PLoS Biol. 2005, 3, e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filatov, D.A.; Moneger, F.; Negrutiu, I.; Charlesworth, D. Low variability in a Y-linked plant gene and its implications for Y-chromosome evolution. Nature 2000, 404, 388–390. [Google Scholar] [CrossRef] [PubMed]
- Miller, P.M.; Kesseli, R.V. A sex-chromosome mutation in Silene latifolia. Sex. Plant Reprod. 2011, 24, 211–217. [Google Scholar] [CrossRef] [PubMed]
- Zluvova, J.; Zak, J.; Janoušek, B.; Vyskot, B. Dioecious Silene latifolia plants show sexual dimorphism in the vegetative stage. BMC Plant Biol. 2010, 10. [Google Scholar] [CrossRef] [PubMed]
- Delph, L.F.; Arntz, A.M.; Scotti-Saintagne, C.; Scotti, I. The genomic architecture of sexual dimorphism in the dioecious plant Silene latifolia. Evolution 2010, 64, 2873–2886. [Google Scholar] [CrossRef] [PubMed]
- Ruddat, M.; Kokontis, J.; Birch, L.; Garber, E.D.; Chiang, K.S.; Campanella, J.; Dai, H. Interactions of Microbotryum violaceum (Ustilago violacea) with its host plant Silene alba. Plant Sci. 1991, 80, 157–165. [Google Scholar] [CrossRef]
- Uchida, W.; Matsunaga, S.; Sugiyama, R.; Kazama, Y.; Kawano, S. Morphological development of anthers induced by the dimorphic smut fungus Microbotryum violaceum in female flowers of the dioecious plant Silene latifolia. Planta 2003, 218, 240–248. [Google Scholar] [CrossRef] [PubMed]
- Uchida, W.; Matsunaga, S.; Kawano, S. Ultrastructural analysis of the behavior of the dimorphic fungus Microbotryum violaceum in fungus-induced anthers of female Silene latifolia flowers. Protoplasma 2005, 226, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Shykoff, J.A.; Bucheli, E. Pollinator visitation patterns, floral rewards and the probability of transmission of Microbotryum violaceum, a veneral disease of plants. J. Ecol. 1995, 83, 189–198. [Google Scholar] [CrossRef]
- Jennersten, O. Butterfly visitors as vectors of Ustilago violacea spores between caryophyllaceous plants. Oikos 1983, 40, 125–130. [Google Scholar] [CrossRef]
- Garber, E.D.; Day, A.W. Genetic mapping of a phytopathogenic basidiomycete, Ustilago violacea. Bot. Gaz. 1985, 146, 449–459. [Google Scholar] [CrossRef]
- Cummins, J.E.; Day, A.W. Genetic and cell cycle analysis of a smut fungus (Ustilago violacea). Methods Cell. Biol. 1977, 15, 445–469. [Google Scholar] [PubMed]
- Schäfer, A.M.; Kemler, M.; Bauer, R.; Begerow, D. The illustrated life cycle of Microbotryum on the host plant Silene latifolia. Botany 2010, 88, 875–885. [Google Scholar] [CrossRef]
- Warmke, H.E. Sex determination and sex balance in Melandrium. Am. J. Bot. 1946, 33, 648–660. [Google Scholar] [CrossRef]
- Warmke, H.E.; Blakeslee, A.F. Sex mechanism in polyploids of Melandrium. Science 1939, 89, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Westergaard, M. Aberrant Y chromosomes and sex expression in Melandium album. Hereditas 1946, 32, 419–443. [Google Scholar] [CrossRef] [PubMed]
- Grant, S.; Hunkirchen, B.; Saedler, H. Developmental differences between male and female flowers in the dioecious plant Silene latifolia. Plant J. 1994, 6, 471–480. [Google Scholar] [CrossRef]
- Zemp, N.; Minder, A.; Widmer, A. Identification of internal reference genes for gene expression normalization between the two sexes in dioecious White Campion. PLoS ONE 2014, 9, e92893. [Google Scholar] [CrossRef] [PubMed]
- Moccia, M.D.; Oger-Desfeux, C.; Marais, G.A.; Widmer, A. A White Campion (Silene latifolia) floral expressed sequence tag (EST) library: Annotation, EST-SSR characterization, transferability, and utility for comparative mapping. BMC Genom. 2009, 10. [Google Scholar] [CrossRef] [PubMed]
- Muyle, A.; Zemp, N.; Deschamps, C.; Mousset, S.; Widmer, A.; Marais, G.A. Rapid de novo evolution of X chromosome dosage compensation in Silene latifolia, a plant with young sex chromosomes. PLoS Biol. 2012, 10, e1001308. [Google Scholar] [CrossRef] [PubMed]
- Hardenack, S.; Ye, D.; Saedler, H.; Grant, S. Comparison of MADS box gene expression in developing male and female flowers of the dioecious plant white campion. Plant Cell. 1994, 6, 1775–1787. [Google Scholar] [CrossRef] [PubMed]
- Ageez, A.; Kazama, Y.; Sugiyama, R.; Kawano, S. Male-fertility genes expressed in male flower buds of Silene latifolia include homologs of anther-specific genes. Genes Genet. Syst. 2005, 80, 403–413. [Google Scholar] [CrossRef] [PubMed]
- Perlin, M.H.; Amselem, J.; Fontanillas, E.; Toh, S.S.; Chen, Z.; Goldberg, J.; Duplessis, S.; Henrissat, B.; Young, S.; Zeng, Q.; et al. Sex and parasites: Genomic and transcriptomic analysis of Microbotryum lychnidis-dioicae, the biotrophic and plant-castrating anther smut fungus. BMC Genom. 2015, 16. [Google Scholar] [CrossRef] [Green Version]
- Toh, S.S.; Chen, Z.; Cuomo, C.; Perlin, M.H. Pas de deux: An intricate dance of anther smut and its host. 2015. In preparation. [Google Scholar]
- Farbos, I.; Oliveira, M.; Negrutiu, I.; Mouras, A. Sex organ determination and differentiation in the dioecious plant Melandrium album (Silene latifolia): A cytological and histological analysis. Sex. Plant Reprod. 1997, 10, 155–167. [Google Scholar] [CrossRef]
- Biere, A.; Honders, S. Host adaptation in the anther smut fungus Ustilago violacea (Microbotryum violaceum): Infection success, spore production and alteration of floral traits on two host species and their F1-hybrid. Oecologia 1996, 107, 307–320. [Google Scholar] [CrossRef]
- Shykoff, J.A.; Kaltz, O. Effects of the anther smut fungus Microbotryum violaceum on host life history patterns in Silene latifolia (Caryophyllaceae). Int. J. Plant. Sci. 1997, 158, 164–171. [Google Scholar] [CrossRef]
- Fujita, N.; Aonuma, W.; Shimizu, Y.; Yamanaka, K.; Hirata, A.; Hood, M.E.; Kawano, S. A petalless flower caused by a Microbotryum violaceum mutant. Int. J. Plant Sci. 2012, 173, 464–473. [Google Scholar] [CrossRef]
- Hood, M.E. Dynamics of multiple infection and within-host competition by the anther-smut pathogen. Am. Nat. 2003, 162, 122–133. [Google Scholar] [CrossRef] [PubMed]
- Akhter, S.; Antonovics, J. Use of internal transcribed spacer primers and fungicide treatments to study the anther-smut disease, Microbotryum violaceum (= Ustilago violacea), of White Campion Silene alba (= Silene latifolia). Int. J. Plant. Sci. 1999, 160, 1171–1176. [Google Scholar] [CrossRef] [PubMed]
- Gibson, A.K.; Petit, E.; Mena-Ali, J.; Oxelman, B.; Hood, M.E. Life-history strategy defends against disease and may select against physiological resistance. Ecol. Evol. 2013, 3, 1741–1750. [Google Scholar] [CrossRef] [PubMed]
- Shykoff, J.A.; Kaltz, O. Phenotypic changes in host plants diseased by Microbotryum violaceum: Parasite manipulation, side effects, and trade-offs. Int. J. Plant Sci. 1998, 159, 236–243. [Google Scholar] [CrossRef]
- Hood, M. Infection rate of Microbotryum lychnidis-dioicae Lamole strains. Personal communication, 2015. [Google Scholar]
- Granberg, Å. Microbotryum violaceum on Silene dioica—Understanding traits that influence plant-pathogen interactions. Ph.D. Thesis, Umeå University, Umeå, Sweden, 2007. [Google Scholar]
- Antonovics, J.; Hood, M.; Partain, J. The ecology and genetics of a host shift: Microbotryum as a model system. Am. Nat. 2002, 160, S40–S53. [Google Scholar] [CrossRef] [PubMed]
- Carlsson-Graner, U.; Pettersson, T.M. Host susceptibility and spread of disease in a metapopulation of Silene dioica. Evol. Ecol. Res. 2005, 7, 353–369. [Google Scholar]
- Schroeder, A.; Mueller, O.; Stocker, S.; Salowsky, R.; Leiber, M.; Gassmann, M.; Lightfoot, S.; Menzel, W.; Granzow, M.; Ragg, T. The RIN: An RNA integrity number for assigning integrity values to RNA measurements. BMC Mol. Biol. 2006, 7. [Google Scholar] [CrossRef] [PubMed]
- Hood, M.E.; Antonovics, J.; Koskella, B. Shared forces of sex chromosome evolution in haploid-mating and diploid-mating organisms: Microbotryum violaceum and other model organisms. Genetics 2004, 168, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Alizon, S.; Hurford, A.; Mideo, N.; Van Baalen, M. Virulence evolution and the trade-off hypothesis: History, current state of affairs and the future. J. Evol. Biol. 2009, 22, 245–259. [Google Scholar] [CrossRef] [PubMed]
- Smith, J. Superinfection drives virulence evolution in experimental populations of bacteria and plasmids. Evolution 2011, 65, 831–841. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toh, S.S.; Perlin, M.H. Size Does Matter: Staging of Silene latifolia Floral Buds for Transcriptome Studies. Int. J. Mol. Sci. 2015, 16, 22027-22045. https://doi.org/10.3390/ijms160922027
Toh SS, Perlin MH. Size Does Matter: Staging of Silene latifolia Floral Buds for Transcriptome Studies. International Journal of Molecular Sciences. 2015; 16(9):22027-22045. https://doi.org/10.3390/ijms160922027
Chicago/Turabian StyleToh, Su San, and Michael H. Perlin. 2015. "Size Does Matter: Staging of Silene latifolia Floral Buds for Transcriptome Studies" International Journal of Molecular Sciences 16, no. 9: 22027-22045. https://doi.org/10.3390/ijms160922027
APA StyleToh, S. S., & Perlin, M. H. (2015). Size Does Matter: Staging of Silene latifolia Floral Buds for Transcriptome Studies. International Journal of Molecular Sciences, 16(9), 22027-22045. https://doi.org/10.3390/ijms160922027







