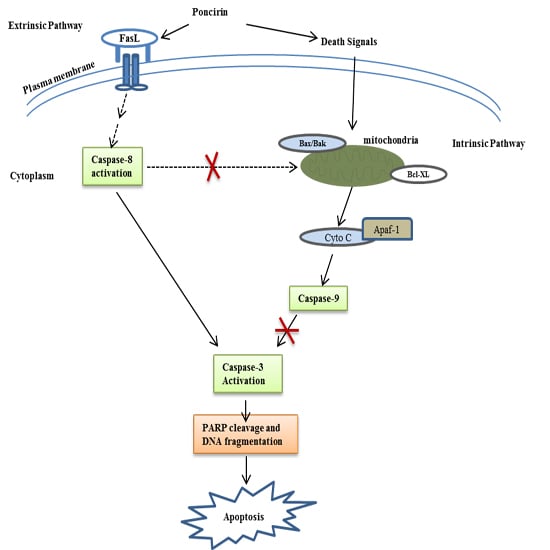
Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand
Abstract
:1. Introduction

2. Results
2.1. Poncirin Inhibits Proliferation of AGS Human Gastric Cancer Cells
2.2. Poncirin Induced Sub-G1 Accumulation and Apoptosis in AGS Cells


2.3. Poncirin Induced Apoptosis via FasL Dependent Extrinsic Apoptotic Pathway in AGS Gastric Cancer Cells


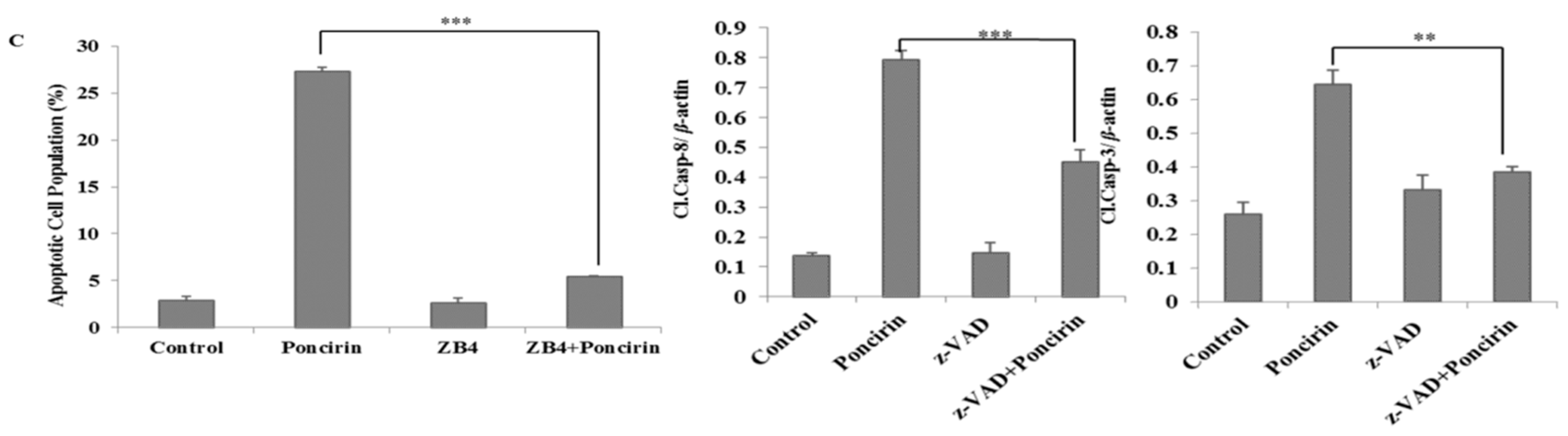
2.4. Poncirin Induced Apoptotic Cell Death Is Independent of Intrinsic Mitochondrial Apoptotic Pathway in AGS Cells


3. Discussion
4. Material and Methods
4.1. Chemicals and Reagents
4.2. Cell Viability Assay
4.3. Flow Cytometry Analysis for Cell Cycle Analysis
4.4. Annexin V-Propidium Iodide Apoptosis Detection
4.5. Nuclear Staining with DAPI
4.6. DNA Fragmentation Assay
4.7. Inhibitor Assay
4.8. Measurement of Mitochondrial Membrane Potential (MMP, ΔΨm)
4.9. Western Blot Analysis

4.10. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crew, K.D.; Neugut, A.I. Epidemiology of gastric cancer. World J. Gastroenterol. 2006, 12, 354–362. [Google Scholar] [PubMed]
- Green, D.; Ponce de Leon, S.; Leon-Rodriguez, E.; Sosa-Sanchez, R. Adenocarcinoma of the stomach: Univariate and multivariate analysis of factors associated with survival. Am. J. Clin. Oncol. 2002, 25, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Harrison, L.E.; Karpeh, M.S.; Brennan, M.F. Extended lymphadenectomy is associated with a survival benefit for node-negative gastric cancer. J. Gastrointest. Surg. 1998, 2, 126–131. [Google Scholar] [CrossRef]
- Jung, K.W.; Won, Y.J.; Kong, H.J.; Oh, C.M.; Lee, D.H.; Lee, J.S. Cancer statistics in Korea: Incidence, mortality, survival, and prevalence in 2011. Cancer Res. Treat. 2014, 46, 109–123. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.E.; Mannisto, S.; Spiegelman, D.; Hunter, D.J.; Bernstein, L.; van den Brandt, P.A.; Buring, J.E.; Cho, E.; English, D.R.; Flood, A.; et al. Intakes of fruit, vegetables, and carotenoids and renal cell cancer risk: A pooled analysis of 13 prospective studies. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1730–1739. [Google Scholar] [CrossRef] [PubMed]
- Gandini, S.; Merzenich, H.; Robertson, C.; Boyle, P. Meta-analysis of studies on breast cancer risk and diet: The role of fruit and vegetable consumption and the intake of associated micronutrients. Eur. J. Cancer 2000, 36, 636–646. [Google Scholar] [CrossRef]
- Theodoratou, E.; Kyle, J.; Cetnarskyj, R.; Farrington, S.M.; Tenesa, A.; Barnetson, R.; Porteous, M.; Dunlop, M.; Campbell, H. Dietary flavonoids and the risk of colorectal cancer. Cancer Epidemiol. Biomark. Prev. 2007, 16, 684–693. [Google Scholar] [CrossRef] [PubMed]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.I.; Lee, S.J.; Lee, S.B.; Park, K.; Kim, W.J.; Moon, S.K. Requirement for Ras/Raf/ERK pathway in naringin-induced G1-cell-cycle arrest via p21WAF1 expression. Carcinogenesis 2008, 29, 1701–1709. [Google Scholar] [CrossRef] [PubMed]
- Luo, G.; Guan, X.; Zhou, L. Apoptotic effect of citrus fruit extract nobiletin on lung cancer cell line a549 in vitro and in vivo. Cancer Biol. Ther. 2008, 7, 966–973. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.B.; Han, A.R.; Park, E.Y.; Kim, J.Y.; Cho, W.; Lee, J.; Seo, E.K.; Lee, K.T. Inhibition of LPS-induced iNOS, COX-2 and cytokines expression by Poncirin through the NF-κB inactivation in raw 264.7 macrophage cells. Biol. Pharm. Bull. 2007, 30, 2345–2351. [Google Scholar] [CrossRef] [PubMed]
- Yoon, H.Y.; Won, Y.Y.; Chung, Y.S. Poncirin prevents bone loss in glucocorticoid-induced osteoporosis in vivo and in vitro. J. Bone Miner. Metab. 2012, 30, 509–516. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Luo, F.; Zheng, Y.; Zhang, J.; Huang, J.; Sun, C.; Li, X.; Chen, K. Characterization, purification of Poncirin from edible Citrus ougan (Citrus reticulate cv. Suavissima) and its growth inhibitory effect on human gastric cancer cells SGC-7901. Int. J. Mol. Sci. 2013, 14, 8684–8697. [Google Scholar] [CrossRef] [PubMed]
- Buendia, B.; Santa-Maria, A.; Courvalin, J.C. Caspase-dependent proteolysis of integral and peripheral proteins of nuclear membranes and nuclear pore complex proteins during apoptosis. J. Cell Sci. 1999, 112, 1743–1753. [Google Scholar] [PubMed]
- Kerr, J.F.; Wyllie, A.H.; Currie, A.R. Apoptosis: A basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 1972, 26, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Nijhawan, D.; Budihardjo, I.; Srinivasula, S.M.; Ahmad, M.; Alnemri, E.S.; Wang, X. Cytochrome c and dATP-dependent formation of Apaf-1/caspase-9 complex initiates an apoptotic protease cascade. Cell 1997, 91, 479–489. [Google Scholar] [CrossRef]
- Walczak, H.; Krammer, P.H. The CD95 (APO-1/Fas) and the TRAIL (APO-2L) apoptosis systems. Exp. Cell Res. 2000, 256, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zhou, Y.; Cheng, X.; Fan, Y.; He, S.; Li, S.; Ye, H.; Xie, C.; Wu, W.; Li, C.; et al. Isogambogenic acid induces apoptosis-independent autophagic cell death in human non-small-cell lung carcinoma cells. Sci. Rep. 2015, 5, 7697. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.F.; Yang, J.S.; Chang, W.S.; Tsai, S.C.; Peng, S.F.; Zhou, Y.R. Houttuynia cordata thunb extract modulates G0/G1 arrest and Fas/CD95-mediated death receptor apoptotic cell death in human lung cancer A549 cells. J. Biomed. Sci. 2013, 20, 18. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Mahfouz, R.Z.; Sharma, R.K.; Sarkar, O.; Mangrola, D.; Mathur, P.P. Potential biological role of poly (ADP-ribose) polymerase (PARP) in male gametes. Reprod. Biol. Endocrinol. 2009, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Raha, S.; Yumnam, S.; Hong, G.E.; Lee, H.J.; Saralamma, V.V.; Park, H.S.; Heo, J.D.; Lee, S.J.; Kim, E.H.; Kim, J.A.; et al. Naringin induces autophagy-mediated growth inhibition by downregulating the PI3K/Akt/mTOR cascade via activation of mapk pathways in ags cancer cells. Int. J. Oncol. 2015, 47, 1061–1069. [Google Scholar] [CrossRef] [PubMed]
- Yumnam, S.; Park, H.S.; Kim, M.K.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Lee, W.S.; Kim, E.H.; Cho, J.H.; Shin, S.C.; et al. Hesperidin induces paraptosis like cell death in hepatoblatoma, HepG2 cells: Involvement of ERK1/2 MAPK. PLoS ONE 2014, 9, e101321. [Google Scholar] [CrossRef] [PubMed]
- Hsiao, P.C.; Lee, W.J.; Yang, S.F.; Tan, P.; Chen, H.Y.; Lee, L.M.; Chang, J.L.; Lai, G.M.; Chow, J.M.; Chien, M.H. Nobiletin suppresses the proliferation and induces apoptosis involving MAPKs and caspase-8/-9/-3 signals in human acute myeloid leukemia cells. Tumour Biol. 2014, 35, 11903–11911. [Google Scholar] [CrossRef] [PubMed]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Ha, M.; Gong, Y.; Xu, Y.; Dong, N.; Yuan, Y. Allicin induces apoptosis in gastric cancer cells through activation of both extrinsic and intrinsic pathways. Oncol. Rep. 2010, 24, 1585–1592. [Google Scholar] [PubMed]
- Li, X.; Lao, Y.; Zhang, H.; Wang, X.; Tan, H.; Lin, Z.; Xu, H. The natural compound Guttiferone F sensitizes prostate cancer to starvation induced apoptosis via calcium and JNK elevation. BMC Cancer 2015, 15, 254. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Wang, X.; Huang, Y.; Chen, Y.; Zhao, G.; Yao, Q.; Jin, C.; Liu, X.; Li, G. Down-regulated SOX4 expression suppresses cell proliferation, metastasis and induces apoptosis in xuanwei female lung cancer patients. J. Cell. Biochem. 2015, 116, 1007–1018. [Google Scholar] [CrossRef] [PubMed]
- Majno, G.; Joris, I. Apoptosis, oncosis, and necrosis. An overview of cell death. Am. J. Pathol. 1995, 146, 3–15. [Google Scholar] [PubMed]
- Hyun, H.B.; Lee, W.S.; Go, S.I.; Nagappan, A.; Park, C.; Han, M.H.; Hong, S.H.; Kim, G.; Kim, G.Y.; Cheong, J.; et al. The flavonoid morin from moraceae induces apoptosis by modulation of Bcl-2 family members and Fas receptor in HCT 116 cells. Int J. Oncol. 2015, 46, 2670–2678. [Google Scholar] [CrossRef] [PubMed]
- Park, H.S.; Park, K.I.; Lee, D.H.; Kang, S.R.; Nagappan, A.; Kim, J.A.; Kim, E.H.; Lee, W.S.; Shin, S.C.; Hah, Y.S.; et al. Polyphenolic extract isolated from korean Lonicera japonica Thunb. Induce G2/M cell cycle arrest and apoptosis in HepG2 cells: Involvements of PI3K/AKT and MAPKs. Food Chem. Toxicol. 2012, 50, 2407–2416. [Google Scholar] [CrossRef] [PubMed]
- Lazebnik, Y.A.; Kaufmann, S.H.; Desnoyers, S.; Poirier, G.G.; Earnshaw, W.C. Cleavage of poly(ADP-ribose) polymerase by a proteinase with properties like ICE. Nature 1994, 371, 346–347. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Seo, H.S.; Kim, J.H.; Um, J.Y.; Shin, Y.C.; Ko, S.G. Ethanol extract of Paeonia suffruticosa andrews (PSE) induced AGS human gastric cancer cell apoptosis via fas-dependent apoptosis and MDM2-p53 pathways. J. Biomed. Sci. 2012, 19, 82. [Google Scholar] [CrossRef] [PubMed]
- Saxena, N.; Yadav, P.; Kumar, O. The Fas/Fas ligand apoptotic pathway is involved in abrin-induced apoptosis. Toxicol. Sci. 2013, 135, 103–118. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Liu, X.; Bhalla, K.; Kim, C.N.; Ibrado, A.M.; Cai, J.; Peng, T.I.; Jones, D.P.; Wang, X. Prevention of apoptosis by Bcl-2: Release of cytochrome c from mitochondria blocked. Science 1997, 275, 1129–1132. [Google Scholar] [CrossRef] [PubMed]
- Mouria, M.; Gukovskaya, A.S.; Jung, Y.; Buechler, P.; Hines, O.J.; Reber, H.A.; Pandol, S.J. Food-derived polyphenols inhibit pancreatic cancer growth through mitochondrial cytochrome c release and apoptosis. Int. J. Cancer 2002, 98, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Han, M.H.; Lee, W.S.; Jung, J.H.; Jeong, J.H.; Park, C.; Kim, H.J.; Kim, G.; Jung, J.M.; Kwon, T.K.; Kim, G.Y.; et al. Polyphenols isolated from Allium cepa L. induces apoptosis by suppressing IAP-1 through inhibiting PI3K/Akt signaling pathways in human leukemic cells. Food Chem. Toxicol. 2013, 62, 382–389. [Google Scholar] [CrossRef] [PubMed]
- Sinha, K.; Das, J.; Pal, P.B.; Sil, P.C. Oxidative stress: The mitochondria-dependent and mitochondria-independent pathways of apoptosis. Arch. Toxicol. 2013, 87, 1157–1180. [Google Scholar] [CrossRef] [PubMed]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saralamma, V.V.G.; Nagappan, A.; Hong, G.E.; Lee, H.J.; Yumnam, S.; Raha, S.; Heo, J.D.; Lee, S.J.; Lee, W.S.; Kim, E.H.; et al. Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand. Int. J. Mol. Sci. 2015, 16, 22676-22691. https://doi.org/10.3390/ijms160922676
Saralamma VVG, Nagappan A, Hong GE, Lee HJ, Yumnam S, Raha S, Heo JD, Lee SJ, Lee WS, Kim EH, et al. Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand. International Journal of Molecular Sciences. 2015; 16(9):22676-22691. https://doi.org/10.3390/ijms160922676
Chicago/Turabian StyleSaralamma, Venu Venkatarame Gowda, Arulkumar Nagappan, Gyeong Eun Hong, Ho Jeong Lee, Silvia Yumnam, Suchismita Raha, Jeong Doo Heo, Sang Joon Lee, Won Sup Lee, Eun Hee Kim, and et al. 2015. "Poncirin Induces Apoptosis in AGS Human Gastric Cancer Cells through Extrinsic Apoptotic Pathway by up-Regulation of Fas Ligand" International Journal of Molecular Sciences 16, no. 9: 22676-22691. https://doi.org/10.3390/ijms160922676