Urine Levels of Defensin α1 Reflect Kidney Injury in Leptospirosis Patients
Abstract
:1. Introduction
2. Results
2.1. Characteristics of the Study Population
2.2. Detection of Urinary Markers in Leptospirosis Patients
2.3. Urinary DA1, NGAL, and NAG Concentrations in Leptospirosis Patients
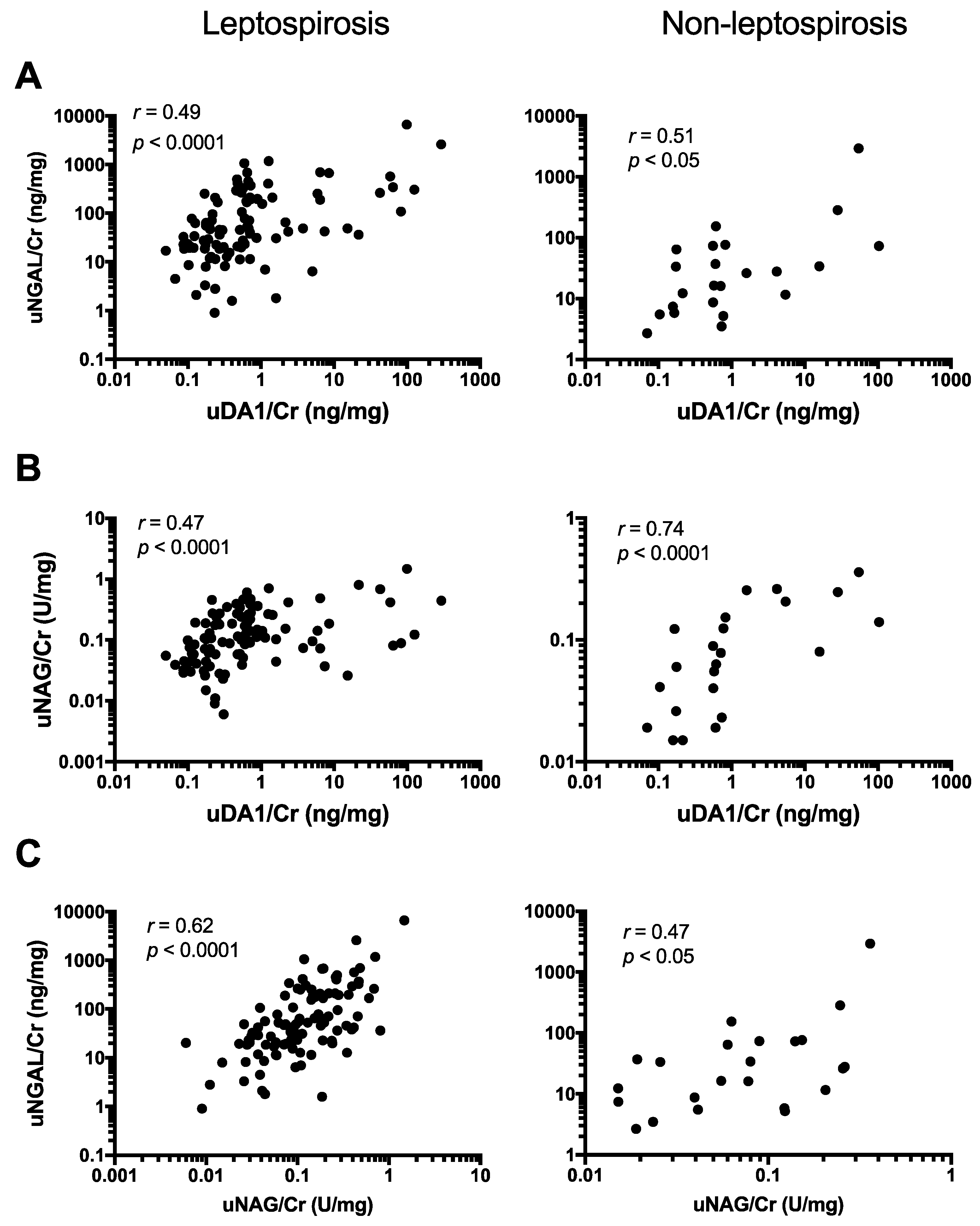
2.4. uDA1/Cr Levels Were Correlated with uNGAL/Cr and uNAG/Cr Levels in Leptospirosis Patients
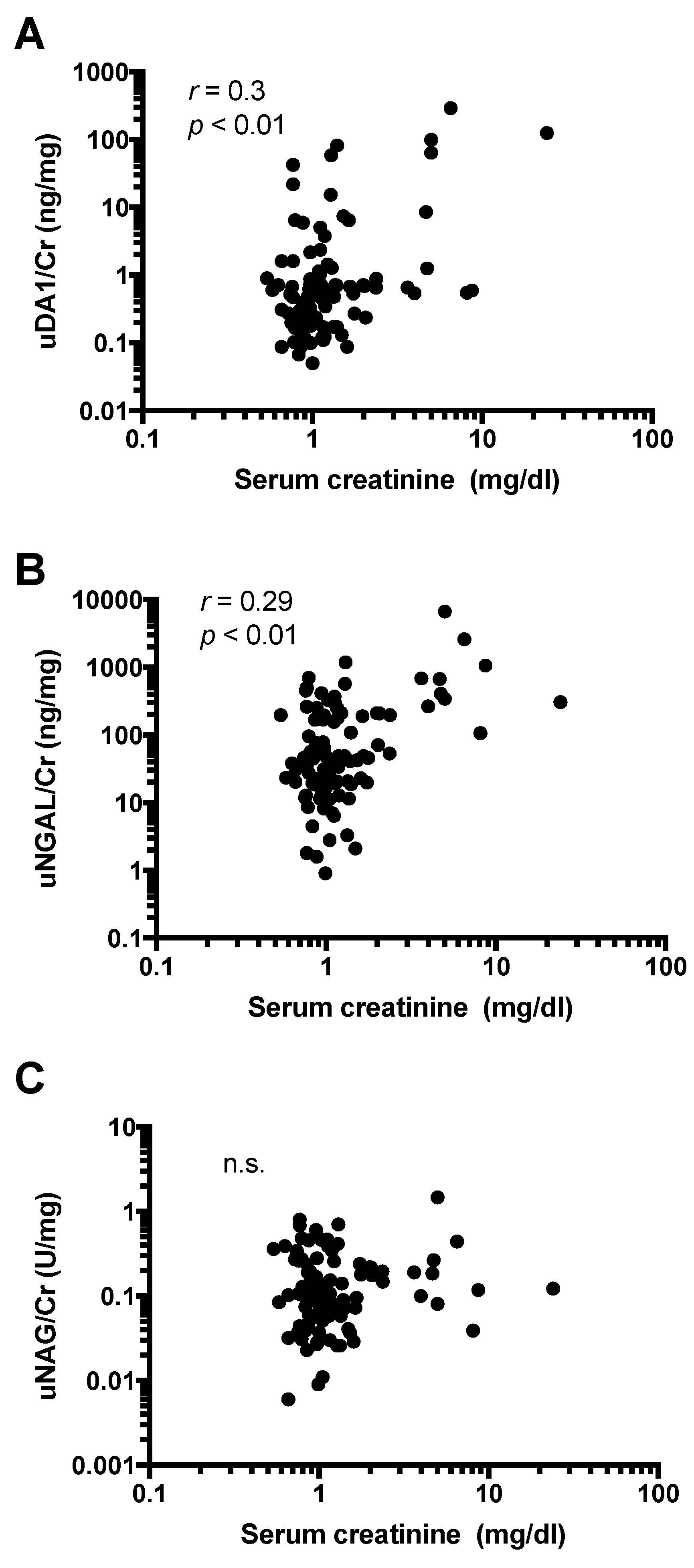
2.5. Urine Levels of DA1 Are Associated with Kidney Injury
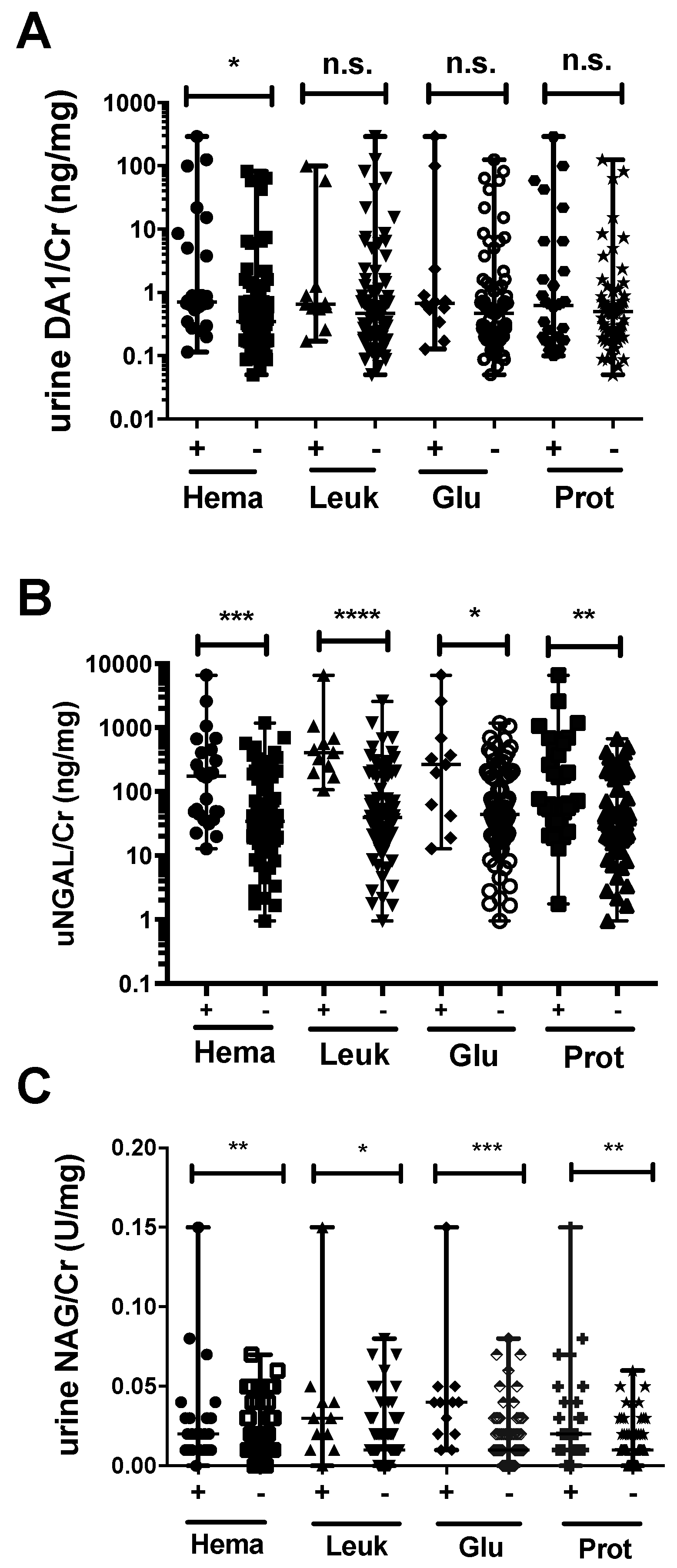
2.6. Association between Urinary Markers and Dipstick Parameters
3. Discussion
4. Materials and Methods
4.1. Study Subjects
4.2. Ethics Statement
4.3. Two-Dimensional Electrophoresis (2-DE) and Mass Spectrometry (MS) Analysis
4.4. Measurement of Urinary Markers and Creatinine
4.5. Dipstick Analysis
4.6. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Levett, P.N.; Haake, D.A. Leptospira species (leptospirosis). In Mandell Douglas and Bennett Principles and Practice of Infectious Diseases; Seventh, E., Ed.; Elsevier: Philadelphia, PA, USA, 2010; pp. 3059–3065. [Google Scholar]
- World Health Organization. Leptospirosis worldwide, 1999. Wkly Epidemiol. Rec. 1999, 74, 237–242. [Google Scholar]
- Amilasan, A.S.; Ujiie, M.; Suzuki, M.; Salva, E.; Belo, M.C.; Koizumi, N.; Yoshimatsu, K.; Schmidt, W.P.; Marte, S.; Dimaano, E.M.; et al. Outbreak of leptospirosis after flood, the Philippines, 2009. Emerg. Infect. Dis. 2012, 18, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, H.; Koizumi, N.; Seto, J.; Ajitsu, S.; Fujii, A.; Takasaki, S.; Yamakage, S.; Aoki, S.; Nakayama, K.; Ashino, Y.; et al. Leptospirosis in the tohoku region: Re-emerging infectious disease. Tohoku J. Exp. Med. 2015, 236, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef] [PubMed]
- Gancheva, G. Renal involvement in leptospirosis. J. IMAB 2012, 18, 265–269. [Google Scholar] [CrossRef]
- Yang, C.W.; Wu, M.S.; Pan, M.J. Leptospirosis renal disease. Nephrol. Dial. Transplant. 2001, 16, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.Y.; Yen, T.H.; Lin, C.Y.; Chen, Y.C.; Pan, M.J.; Lee, C.H.; Yu, C.C.; Wu, M.S.; Wu, S.S.; Weng, C.H.; et al. Early identification of leptospirosis as an ignored cause of multiple organ dysfunction syndrome. Shock 2012, 38, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Phimda, K.; Hoontrakul, S.; Suttinont, C.; Chareonwat, S.; Losuwanaluk, K.; Chueasuwanchai, S.; Chierakul, W.; Suwancharoen, D.; Silpasakorn, S.; Saisongkorh, W.; et al. Doxycycline versus azithromycin for treatment of leptospirosis and scrub typhus. Antimicrob Agents Chemother 2007, 51, 3259–3263. [Google Scholar] [CrossRef] [PubMed]
- Nozoe, K.; Aida, Y.; Fukuda, T.; Sanui, T.; Nishimura, F. Mechanisms of the macrolide-induced inhibition of superoxide generation by neutrophils. Inflammation 2016, 39, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Kanoh, S.; Rubin, B.K. Mechanisms of action and clinical application of macrolides as immunomodulatory medications. Clin. Microbiol. Rev. 2010, 23, 590–615. [Google Scholar] [CrossRef] [PubMed]
- Srisawat, N.; Praditpornsilpa, K.; Patarakul, K.; Techapornrung, M.; Daraswang, T.; Sukmark, T.; Khositrangsikun, K.; Fakthongyoo, A.; Oranrigsupak, P.; Praderm, L.; et al. Neutrophil gelatinase associated lipocalin (NGAL) in leptospirosis acute kidney injury: A multicenter study in thailand. PLoS ONE 2015, 10, e0143367. [Google Scholar] [CrossRef] [PubMed]
- Iwasaki, H.; Chagan-Yasutan, H.; Leano, P.S.; Koizumi, N.; Nakajima, C.; Taurustiati, D.; Hanan, F.; Lacuesta, T.L.; Ashino, Y.; Suzuki, Y.; et al. Combined antibody and DNA detection for early diagnosis of leptospirosis after a disaster. Diagn. Microbiol. Infect. Dis. 2016, 84, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Barratt, J.; Topham, P. Urine proteomics: The present and future of measuring urinary protein components in disease. Can. Med. Assoc. J. 2007, 177, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Decramer, S.; Gonzalez de Peredo, A.; Breuil, B.; Mischak, H.; Monsarrat, B.; Bascands, J.L.; Schanstra, J.P. Urine in clinical proteomics. Mol. Cell. Proteom. 2008, 7, 1850–1862. [Google Scholar] [CrossRef] [PubMed]
- Sheira, G.; Noreldin, N.; Tamer, A.; Saad, M. Urinary biomarker N-acetyl-β-d-glucosaminidase can predict severity of renal damage in diabetic nephropathy. J. Diabetes Metab. Disord. 2015, 14. [Google Scholar] [CrossRef] [PubMed]
- Mori, K.; Lee, H.T.; Rapoport, D.; Drexler, I.R.; Foster, K.; Yang, J.; Schmidt-Ott, K.M.; Chen, X.; Li, J.Y.; Weiss, S.; et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J. Clin. Investig. 2005, 115, 610–621. [Google Scholar] [CrossRef] [PubMed]
- Kjeldsen, L.; Johnsen, A.H.; Sengelov, H.; Borregaard, N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J. Biol. Chem. 1993, 268, 10425–10432. [Google Scholar] [PubMed]
- Khositseth, S.; Sudjaritjan, N.; Tananchai, P.; Ong-ajyuth, S.; Sitprija, V.; Thongboonkerd, V. Renal magnesium wasting and tubular dysfunction in leptospirosis. Nephrol. Dial. Transplant. 2008, 23, 952–958. [Google Scholar] [CrossRef] [PubMed]
- Liborio, A.B.; Braz, M.B.; Seguro, A.C.; Meneses, G.C.; Neves, F.M.; Pedrosa, D.C.; Cavalcanti, L.P.; Martins, A.M.; Daher Ede, F. Endothelial glycocalyx damage is associated with leptospirosis acute kidney injury. Am. J. Trop. Med. Hyg. 2015, 92, 611–616. [Google Scholar] [CrossRef] [PubMed]
- Paragas, N.; Kulkarni, R.; Werth, M.; Schmidt-Ott, K.M.; Forster, C.; Deng, R.; Zhang, Q.; Singer, E.; Klose, A.D.; Shen, T.H.; et al. α-intercalated cells defend the urinary system from bacterial infection. J. Clin. Investig. 2014, 124, 2963–2976. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Sevketoglu, E.; Gedikbasi, A.; Karyagar, S.; Kiyak, A.; Mulazimoglu, M.; Aydogan, G.; Ozpacaci, T.; Hatipoglu, S. Early prediction of urinary tract infection with urinary neutrophil gelatinase associated lipocalin. Pediatr. Nephrol. 2009, 24, 2387–2392. [Google Scholar] [CrossRef] [PubMed]
- Lerolle, N.; Nochy, D.; Guerot, E.; Bruneval, P.; Fagon, J.Y.; Diehl, J.L.; Hill, G. Histopathology of septic shock induced acute kidney injury: Apoptosis and leukocytic infiltration. Intensive Care Med. 2010, 36, 471–478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Penna, D.; de Brito, T.; Pupo, A.A.; Machado, M.M.; Ayroza, P.A.; de Almeida, S.S. Kidney biopsy in human leptospirosis. Am. J. Trop. Med. Hyg. 1963, 12, 896–901. [Google Scholar] [PubMed]
- Wang, B.; Sullivan, J.; Sullivan, G.W.; Mandell, G.L. Interaction of leptospires with human polymorphonuclear neutrophils. Infect. Immun. 1984, 44, 459–464. [Google Scholar] [PubMed]
- Dobrina, A.; Nardon, E.; Vecile, E.; Cinco, M.; Patriarca, P. Leptospira icterohemorrhagiae and leptospire peptidolgycans induce endothelial cell adhesiveness for polymorphonuclear leukocytes. Infect. Immun. 1995, 63, 2995–2999. [Google Scholar] [PubMed]
- Schneider, J.J.; Unholzer, A.; Schaller, M.; Schafer-Korting, M.; Korting, H.C. Human defensins. J. Mol. Med. 2005, 83, 587–595. [Google Scholar] [CrossRef] [PubMed]
- Huttner, K.M.; Bevins, C.L. Antimicrobial peptides as mediators of epithelial host defense. Pediatr. Res. 1999, 45, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Xu, L.; Wang, X.; Li, S.; Wang, B. Investigation of microbicidal activity of neutrophil defensins against leptospires. J. West China University Med. Sci. 1992, 23, 126–129. [Google Scholar]
- Bingham, J.; Clarke, H.; Spangehl, M.; Schwartz, A.; Beauchamp, C.; Goldberg, B. The α defensin-1 biomarker assay can be used to evaluate the potentially infected total joint arthroplasty. Clin. Orthop. Relat. Res. 2014, 472, 4006–4009. [Google Scholar] [CrossRef] [PubMed]
- Martensson, J.; Bellomo, R. The rise and fall of NGAL in acute kidney injury. Blood Purif. 2014, 37, 304–310. [Google Scholar] [CrossRef] [PubMed]
- Vanmassenhove, J.; Vanholder, R.; Nagler, E.; van Biesen, W. Urinary and serum biomarkers for the diagnosis of acute kidney injury: An in-depth review of the literature. Nephrol. Dial. Transplant. 2013, 28, 254–273. [Google Scholar] [CrossRef] [PubMed]
- Mishra, J.; Ma, Q.; Prada, A.; Mitsnefes, M.; Zahedi, K.; Yang, J.; Barasch, J.; Devarajan, P. Identification of neutrophil gelatinase-associated lipocalin as a novel early urinary biomarker for ischemic renal injury. J. Am. Soc. Nephrol. 2003, 14, 2534–2543. [Google Scholar] [CrossRef] [PubMed]
- Tsigou, E.; Psallida, V.; Demponeras, C.; Boutzouka, E.; Baltopoulos, G. Role of new biomarkers: Functional and structural damage. Crit. Care Res. Pract. 2013, 2013, 361078. [Google Scholar] [CrossRef] [PubMed]
- Skalova, S. The diagnostic role of urinary N-acetyl-β-d-glucosaminidase (NAG) activity in the detection of renal tubular impairment. Acta Med. 2005, 48, 75–80. [Google Scholar]
- Chang, M.Y.; Cheng, Y.C.; Hsu, S.H.; Ma, T.L.; Chou, L.F.; Hsu, H.H.; Tian, Y.C.; Chen, Y.C.; Sun, Y.J.; Hung, C.C.; et al. Leptospiral outer membrane protein LipL32 induces inflammation and kidney injury in zebrafish larvae. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Villanueva, S.Y.; Ezoe, H.; Baterna, R.A.; Yanagihara, Y.; Muto, M.; Koizumi, N.; Fukui, T.; Okamoto, Y.; Masuzawa, T.; Cavinta, L.L.; et al. Serologic and molecular studies of leptospira and leptospirosis among rats in the philippines. Am. J. Trop. Med. Hyg. 2010, 82, 889–898. [Google Scholar] [CrossRef] [PubMed]
- Koizumi, N.; Nakajima, C.; Harunari, T.; Tanikawa, T.; Tokiwa, T.; Uchimura, E.; Furuya, T.; Mingala, C.N.; Villanueva, M.A.; Ohnishi, M.; et al. A new loop-mediated isothermal amplification method for rapid, simple, and sensitive detection of leptospira spp. In urine. J. Clin. Microbiol. 2012, 50, 2072–2074. [Google Scholar] [CrossRef] [PubMed]
- Ishida, T.; Ichihara, M.; Wang, X.; Yamamoto, K.; Kimura, J.; Majima, E.; Kiwada, H. Injection of PEGylated liposomes in rats elicits PEG-specific IgM, which is responsible for rapid elimination of a second dose of PEGylated liposomes. J. Control. Release 2006, 112, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Kawano, H.; Muto, S.; Ohmoto, Y.; Iwata, F.; Fujiki, H.; Mori, T.; Yan, L.; Horie, S. Exploring urinary biomarkers in autosomal dominant polycystic kidney disease. Clin. Exp. Nephrol. 2014, 19, 968–973. [Google Scholar] [CrossRef] [PubMed]

| Variables | Leptospirosis | Non-Leptospirosis | HC |
|---|---|---|---|
| Demographic data | |||
| Sex: male (n (%)) | 94 (94.9) | 19 (82.6) | 2 (25.0) |
| Age in years (median (range)) | 30 (12–67) | 29 (19–51) | 26 (22–43) |
| Markers (median (range)) | |||
| uDA1/Cr | 0.498 (0.05–292.03) | 0.614 (0.07–103.35) | 0.22 (0.088–0.995) |
| uNGAL/Cr a,c | 46.38 (0.944–6662.4) | 26.23 (2.67–2941.5) | 10.45 (4.04–147.19) |
| uNAG/Cr a,c | 0.107 (0.006–1.469) | 0.077 (0.0151–0.36) | 0.03 (0.008–0.116) |
| Serum Cr a,b,c | 1.01 (0.54–24.04) | 0.855 (0.49–3.71) | 0.69 (0.39–1) |
| Spot Sample ID | 2056 | 2060 | 1648 | 1804 | 1293 |
| Fold Differential (A > B) | 55.3 | 45.7 | 41.7 | 40 | 25 |
| Proteins Matched with Database | neutrophil defensin 1 preproprotein | neutrophil defensin 1 preproprotein | Retinol binding protein 4, plasma | pancreatic stone protein | hemopexin, isoform CRA_a |
| agrin precursor, partial | lipocalin-1 isoform 1 precursor | ||||
| regenerating protein (reg) | prolactin-inducible protein precursor | ||||
| neudesin precursor | |||||
| protein AMBP preproprotein | |||||
| immunoglobulin lambda light chain, partial | |||||
| glia maturation factor gamma | |||||
| SMT3A protein | |||||
| semenogelin | |||||
| DskA-type zinc finger protein (organism: Shewanella piezotolerans WP3) | |||||
| phosphatidylglycerophosphate synthase-like protein (organism: Trypanosoma brucei brucei strain 927/4 GUT at 10.1) |
| Lepto & Non-Lepto | Lepto & Non-lepto, HC | Lepto & HC | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ROC analysis | uDA1/Cr | uNGAL/Cr | uNAG/Cr | Ser Cr | uDA1/Cr | uNGAL/Cr | uNAG/Cr | Ser Cr | uDA1/Cr | uNGAL/Cr | uNAG/Cr | Ser Cr |
| AUC | 0.568 | 0.645 | 0.621 | 0.67 | 0.505 | 0.686 | 0.679 | 0.743 | 0.676 | 0.803 | 0.848 | 0.89 |
| p-value | 0.34 | 0.03 | 0.06 | 0.02 | 0.94 | 0.0008 | 0.001 | <0.0001 | 0.06 | 0.0001 | <0.0001 | <0.0001 |
| Youden index | 0.245 | 0.292 | 0.212 | 0.344 | 0.11 | 0.362 | 0.292 | 0.4 | 0.35 | 0.623 | 0.65 | 0.74 |
| Cut-off | 0.55 | 16.43 | 0.08 | 0.93 | 0.53 | 16.43 | 0.08 | 0.68 | 0.54 | 11.43 | 0.04 | 0.68 |
| Sensitivity (%) | 54.9 | 81.37 | 64.7 | 64.36 | 52.94 | 81.37 | 64.71 | 94.06 | 47.06 | 87.25 | 77.45 | 94.06 |
| Specificity (%) | 69.6 | 47.83 | 56.5 | 70 | 58.06 | 54.84 | 64.52 | 46.67 | 87.5 | 75 | 87.5 | 80 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chagan-Yasutan, H.; Chen, Y.; Lacuesta, T.L.; Leano, P.S.A.; Iwasaki, H.; Hanan, F.; Taurustiati, D.; Ohmoto, Y.; Ashino, Y.; Saitoh, H.; et al. Urine Levels of Defensin α1 Reflect Kidney Injury in Leptospirosis Patients. Int. J. Mol. Sci. 2016, 17, 1637. https://doi.org/10.3390/ijms17101637
Chagan-Yasutan H, Chen Y, Lacuesta TL, Leano PSA, Iwasaki H, Hanan F, Taurustiati D, Ohmoto Y, Ashino Y, Saitoh H, et al. Urine Levels of Defensin α1 Reflect Kidney Injury in Leptospirosis Patients. International Journal of Molecular Sciences. 2016; 17(10):1637. https://doi.org/10.3390/ijms17101637
Chicago/Turabian StyleChagan-Yasutan, Haorile, Yue Chen, Talitha Lea Lacuesta, Prisca Susan A. Leano, Hiroko Iwasaki, Firmanto Hanan, Delsi Taurustiati, Yasukazu Ohmoto, Yugo Ashino, Hiroki Saitoh, and et al. 2016. "Urine Levels of Defensin α1 Reflect Kidney Injury in Leptospirosis Patients" International Journal of Molecular Sciences 17, no. 10: 1637. https://doi.org/10.3390/ijms17101637
APA StyleChagan-Yasutan, H., Chen, Y., Lacuesta, T. L., Leano, P. S. A., Iwasaki, H., Hanan, F., Taurustiati, D., Ohmoto, Y., Ashino, Y., Saitoh, H., Kiyomoto, H., Suzuki, Y., Telan, F. O. E., & Hattori, T. (2016). Urine Levels of Defensin α1 Reflect Kidney Injury in Leptospirosis Patients. International Journal of Molecular Sciences, 17(10), 1637. https://doi.org/10.3390/ijms17101637








