Extracellular Self-DNA (esDNA), but Not Heterologous Plant or Insect DNA (etDNA), Induces Plasma Membrane Depolarization and Calcium Signaling in Lima Bean (Phaseolus lunatus) and Maize (Zea mays)
Abstract
:1. Introduction
2. Results
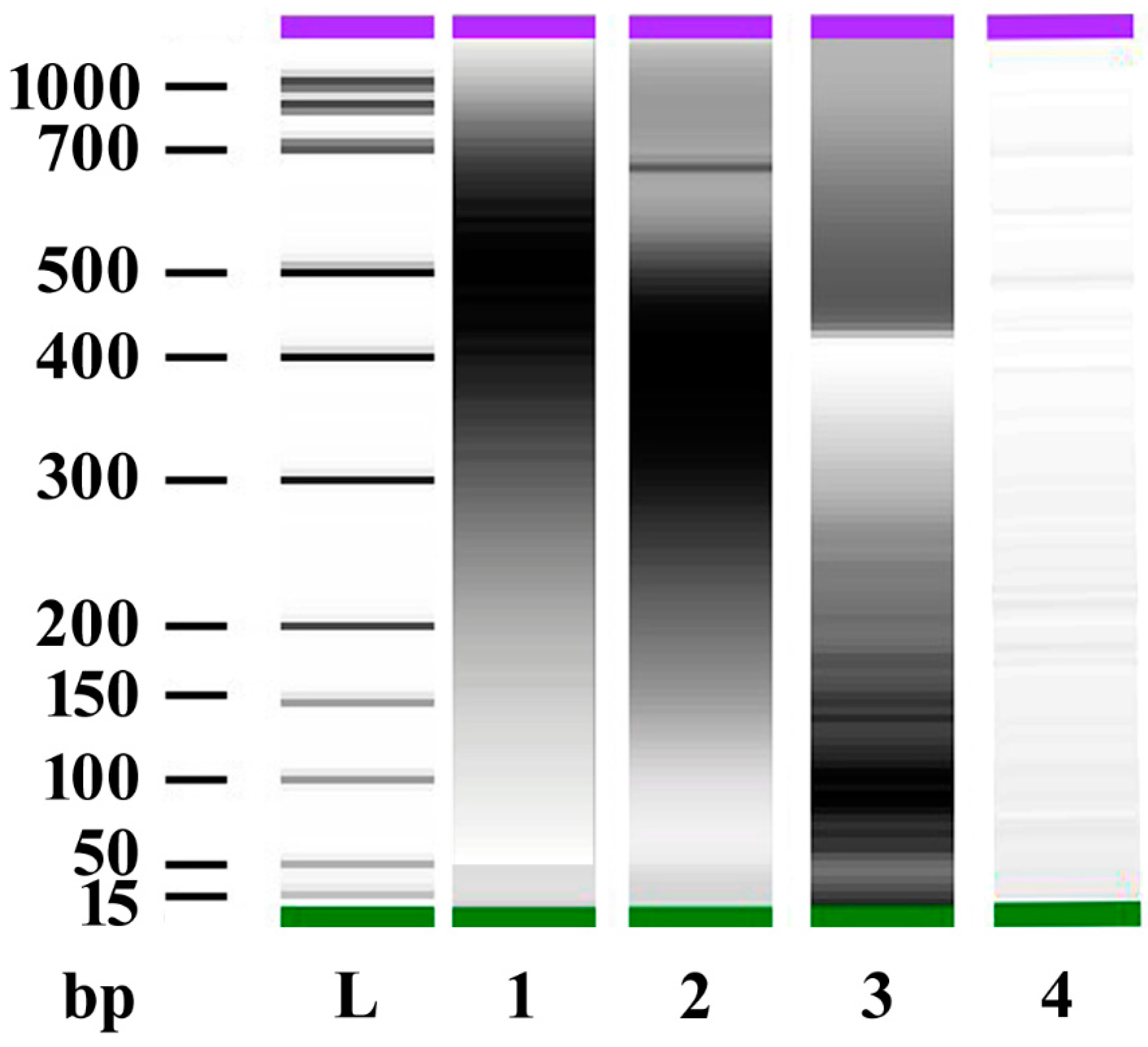
2.1. DNA Sonication and DNA Fragment Analysis
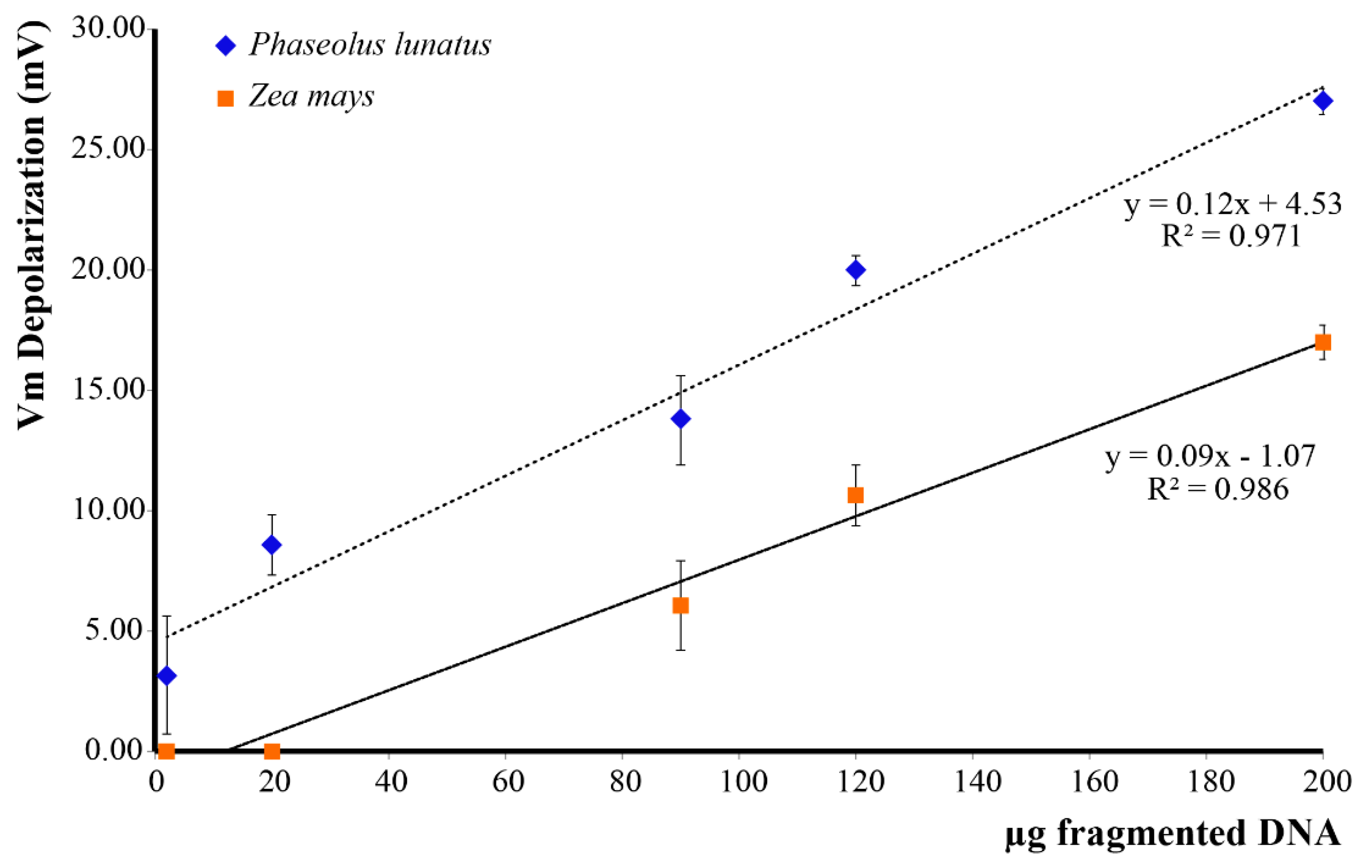
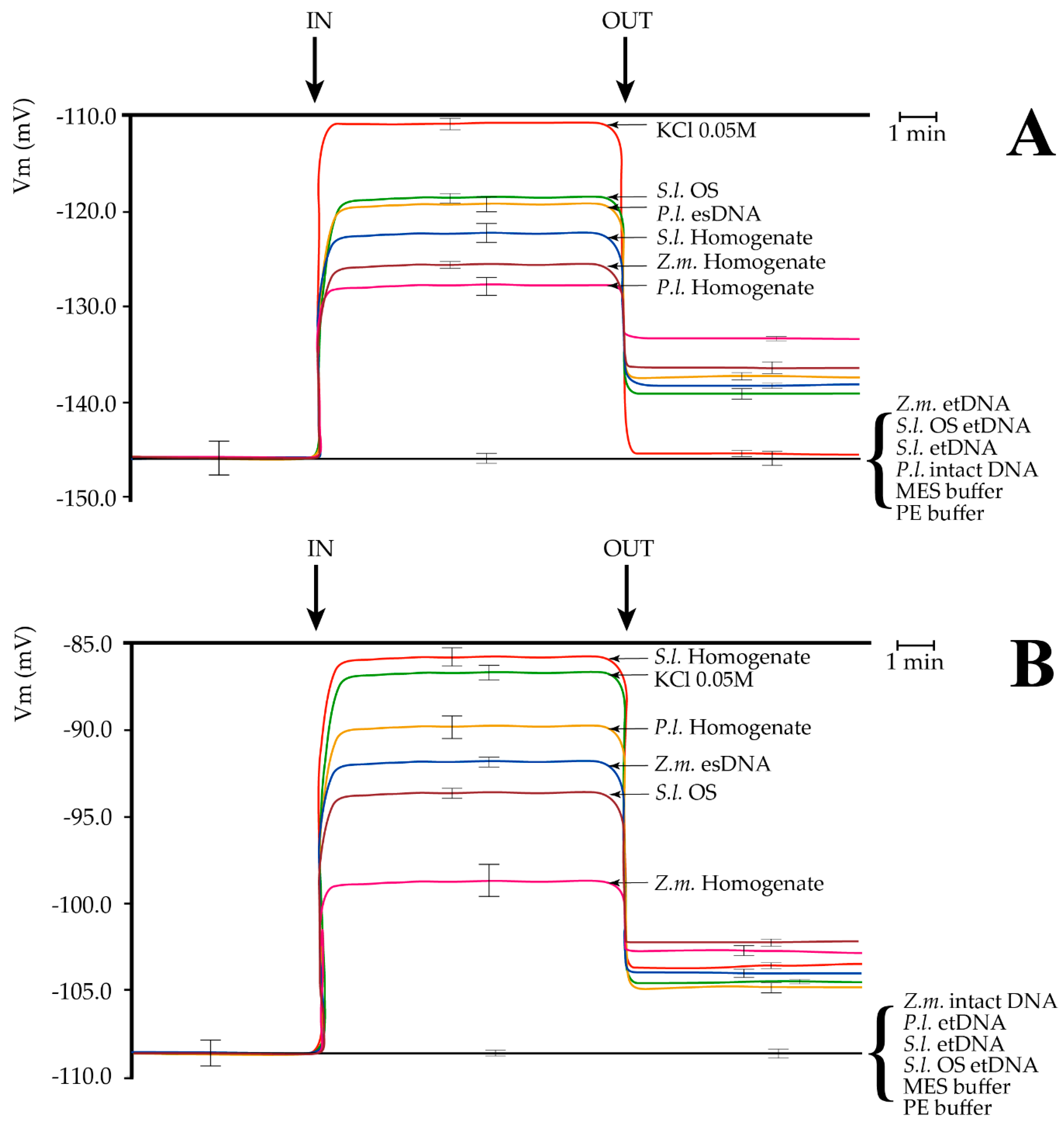
2.2. Extracellular Self-DNA (esDNA) Induces Plasma Membrane Potential (Vm) Depolarization
2.3. Cation Capillary Electrophoresis of Leaf and Larvae Homogenates and of Fragmented DNA
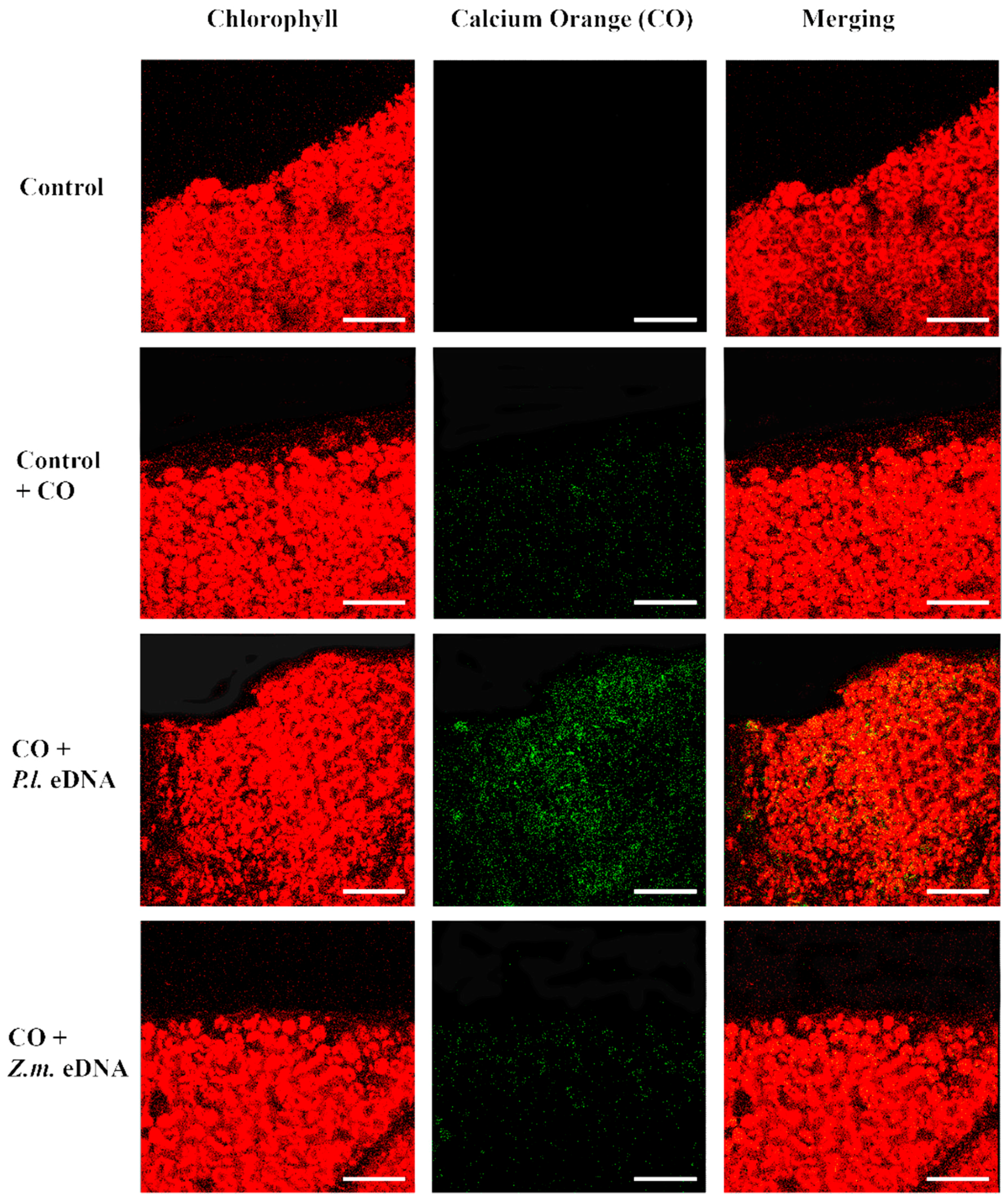
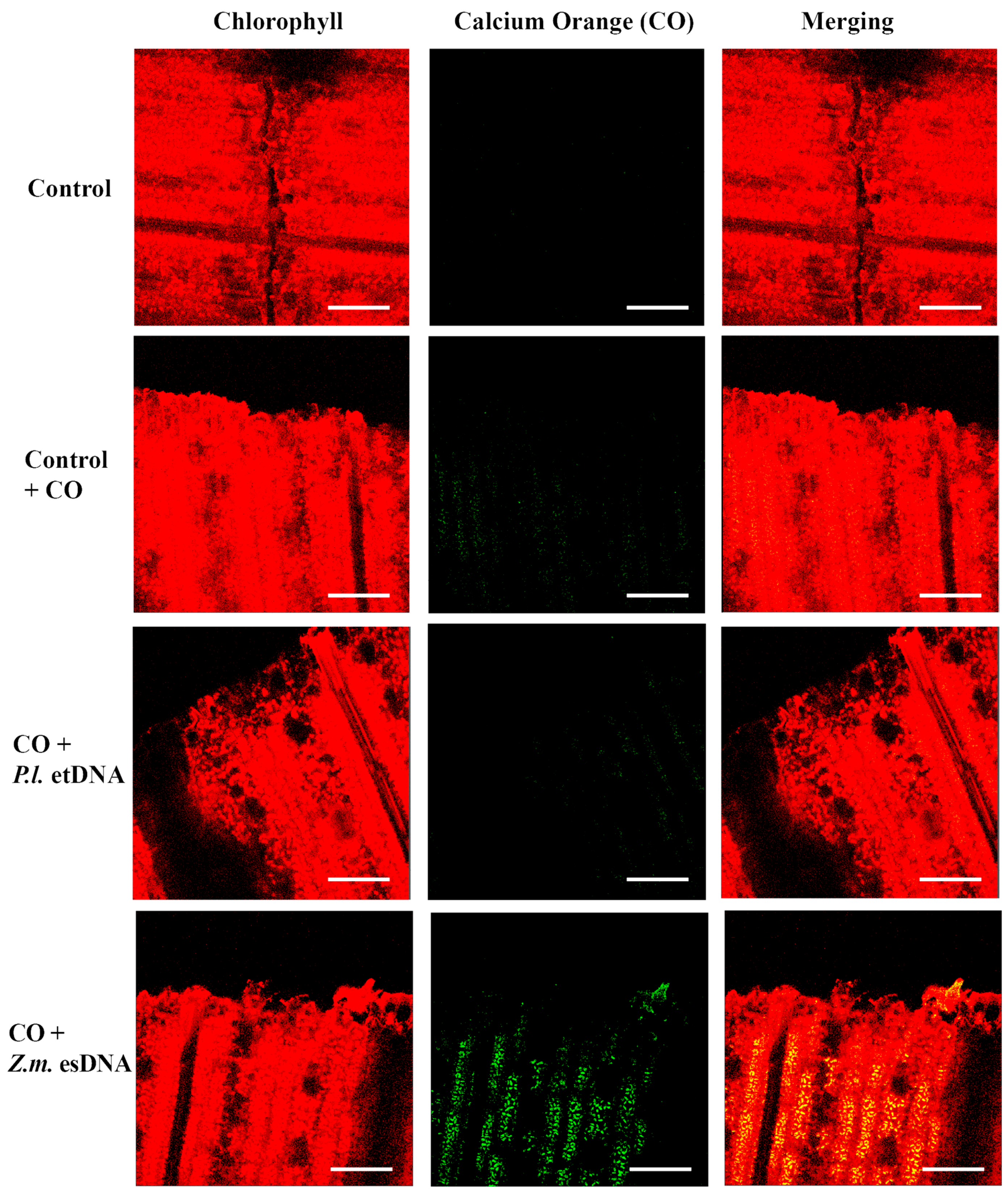
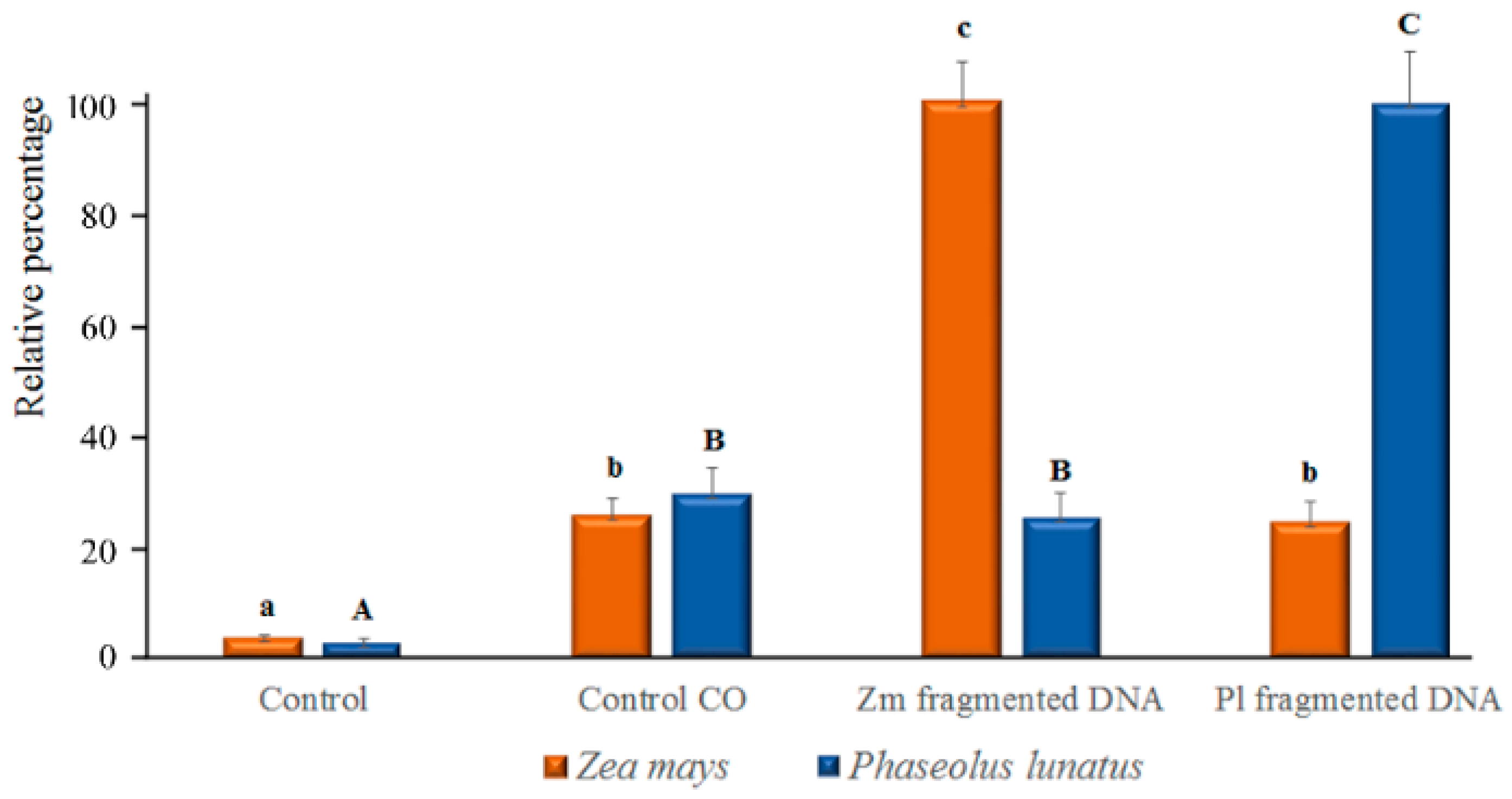
2.4. esDNA Induces Calcium Signaling
3. Discussion
4. Materials and Methods
4.1. Plant and Animal Material
4.2. DNA Extraction and Sonication
4.3. Membrane Potentials
4.4. Determination of Intracellular Calcium Variations Using Confocal Laser Scanning Microscopy (CLSM) and Calcium Orange
4.5. Capillary Electrophoresis and Cation Quantification
4.6. Statistical Analyses
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mazzoleni, S.; Carteni, F.; Bonanomi, G.; Senatore, M.; Termolino, P.; Giannino, F.; Incerti, G.; Rietkerk, M.; Lanzotti, V.; Chiusano, M.L. Inhibitory effects of extracellular self-DNA: A general biological process? New Phytol. 2015, 206, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Mingo, A.; Senatore, M.; Giannino, F.; Carteni, F.; Rietkerk, M.; et al. Inhibitory and toxic effects of extracellular self-DNA in litter: A mechanism for negative plant-soil feedbacks? New Phytol. 2015, 205, 1195–1210. [Google Scholar] [CrossRef] [PubMed]
- Mazzoleni, S.; Carteni, F.; Bonanomi, G.; Incerti, G.; Chiusano, M.L.; Termolino, P.; Migliozzi, A.; Senatore, M.; Giannino, F.; Rietkerk, M.; et al. New perspectives on the use of nucleic acids in pharmacological applications: Inhibitory action of extracellular self-DNA in biological systems. Phytochem. Rev. 2014, 13, 937–946. [Google Scholar] [CrossRef]
- Duran-Flores, D.; Heil, M. Growth inhibition by self-DNA: A phenomenon and its multiple explanations. New Phytol. 2015, 207, 482–485. [Google Scholar] [CrossRef] [PubMed]
- Veresoglou, S.D.; Aguilar-Trigueros, C.A.; Mansour, I.; Rillig, M.C. Self-DNA: A blessing in disguise? New Phytol. 2015, 207, 488–490. [Google Scholar] [CrossRef] [PubMed]
- Duran-Flores, D.; Heil, M. Damaged-self recognition in common bean (Phaseolus vulgaris) shows taxonomic specificity and triggers signaling via reactive oxygen species (ROS). Front. Plant Sci. 2014, 5, 585. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, A.S.; Maffei, M.E. Role of early signalling events in plant–insect interactions. J. Exp. Bot. 2015, 66, 435–448. [Google Scholar] [CrossRef] [PubMed]
- Zebelo, S.A.; Maffei, M.E. Signal transduction in plant–insect interactions: From membrane potential variations to metabolomics. In Plant Electrophysiology; Volkov, A., Ed.; Springer: Berlin, Germany, 2012; pp. 1–30. [Google Scholar]
- Zebelo, S.A.; Matsui, K.; Ozawa, R.; Maffei, M.E. Plasma membrane potential depolarization and cytosolic calcium flux are early events involved in tomato (Solanum lycopersicum) plant-to-plant communication. Plant Sci. 2012, 196, 93–100. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Bossi, S. Electrophysiology and plant responses to biotic stress. In Plant Electrophysiology—Theory and Methods; Volkov, A., Ed.; Springer: Berlin, Germany, 2006; pp. 461–481. [Google Scholar]
- Bricchi, I.; Occhipinti, A.; Bertea, C.M.; Zebelo, S.A.; Brillada, C.; Verrillo, F.; de Castro, C.; Molinaro, A.; Faulkner, C.; Maule, A.J.; et al. Separation of early and late responses to herbivory in Arabidopsis by changing plasmodesmal function. Plant J. 2013, 73, 14–25. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Arimura, G.I.; Uchtenhagen, H.; Bossi, S.; Bertea, C.M.; Cucuzza, L.S.; Novero, M.; Volpe, V.; Quadro, S.; et al. Effects of feeding Spodoptera littoralis on lima bean leaves. III. Membrane depolarization and involvement of hydrogen peroxide. Plant Physiol. 2006, 140, 1022–1035. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Mithöfer, A.; Boland, W. Before gene expression: Early events in plant–insect interaction. Trends Plant Sci. 2007, 12, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Zhou, Q.; Xu, W.; Cai, Y.X.; Yin, Z.N.; Gao, X.M.; Xiong, S.D. DNA-dependent Activator of Interferon-regulatory Factors (DAI) Promotes Lupus Nephritis by Activating the Calcium Pathway. J. Biol. Chem. 2013, 288, 13534–13550. [Google Scholar] [CrossRef] [PubMed]
- Gao, D.X.; Li, T.; Li, X.D.; Chen, X.; Li, Q.Z.; Wight-Carter, M.; Chen, Z.J. Activation of cyclic GMP-AMP synthase by self-DNA causes autoimmune diseases. Proc. Natl. Acad. Sci. USA 2015, 112, E5699–E5705. [Google Scholar] [CrossRef] [PubMed]
- Jakobs, C.; Perner, S.; Hornung, V. AIM2 Drives Joint Inflammation in a Self-DNA Triggered Model of Chronic Polyarthritis. PLoS ONE 2015, 10, e0131702. [Google Scholar] [CrossRef] [PubMed]
- Gilliet, M.; Lande, R. Antimicrobial peptides and self-DNA in autoimmune skin inflammation. Curr. Opin. Immunol. 2008, 20, 401–407. [Google Scholar] [CrossRef] [PubMed]
- Furi, I.; Sipos, F.; Spisak, S.; Kiszner, G.; Wichmann, B.; Scholler, A.; Tulassay, Z.; Muzes, G.; Molnar, B. Association of Self-DNA Mediated TLR9-Related Gene, DNA Methyltransferase, and Cytokeratin Protein Expression Alterations in HT29-Cells to DNA Fragment Length and Methylation Status. Sci. World J. 2013, 2013, 293296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coch, C.; Busch, N.; Wimmenauer, V.; Hartmann, E.; Janke, M.; Abdel-Mottaleb, M.M.A.; Lamprecht, A.; Ludwig, J.; Barchet, W.; Schlee, M.; et al. Higher activation of TLR9 in plasmacytoid dendritic cells by microbial DNA compared with self-DNA based on CpG-specific recognition of phosphodiester DNA. J. Leukoc. Biol. 2009, 86, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Barton, G.M.; Kagan, J.C.; Medzhitov, R. Intracellular localization of Toll-like receptor 9 prevents recognition of self DNA but facilitates access to viral DNA. Nat. Immunol. 2006, 7, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Wakeel, A. Potassium-sodium interactions in soil and plant under saline-sodic conditions. J. Plant Nutr. Soil Sci. 2013, 176, 344–354. [Google Scholar] [CrossRef]
- Maffei, M.; Bossi, S.; Spiteller, D.; Mithöfer, A.; Boland, W. Effects of feeding Spodoptera littoralis on Lima bean leaves. I. Membrane potentials, intracellular calcium variations, oral secretions, and regurgitate components. Plant Physiol. 2004, 134, 1752–1762. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.; Mithofer, A.; Boland, W. Insects feeding on plants: Rapid signals and responses preceding the induction of phytochemical release. Phytochemistry 2007, 68, 22–24. [Google Scholar] [CrossRef] [PubMed]
- Verrillo, F.; Occhipinti, A.; Kanchiswamy, C.N.; Maffei, M.E. Quantitative analysis of herbivore-induced cytosolic calcium by using a Cameleon (YC 3.6) calcium sensor in Arabidopsis thaliana. J. Plant Physiol. 2014, 171, 136–139. [Google Scholar] [CrossRef] [PubMed]
- Kanchiswamy, C.N.; Mohanta, T.K.; Capuzzo, A.; Occhipinti, A.; Verrillo, F.; Maffei, M.E.; Malnoy, M. Role of Ca2+ and CDPKs in resistant and susceptible cultivars of apple (Malus × domestica) in response to the pathogen Erwinia amylovora and mechanical wounding. BMC Genom. 2013, 14, 760. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.; Maffei, M.E. Calcium and secondary CPK signaling in plants in response to herbivore attack. Biochem. Biophys. Res. Commun. 2010, 400, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Kanchiswamy, C.N.; Malnoy, M.; Occhipinti, A.; Maffei, M.E. Calcium imaging perspective in plants. Int. J. Mol. Sci. 2014, 15, 3842–3859. [Google Scholar] [CrossRef] [PubMed]
- Takaoka, A.; Wang, Z.; Choi, M.K.; Yanai, H.; Negishi, H.; Ban, T.; Lu, Y.; Miyagishi, M.; Kodama, T.; Honda, K.; et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature 2007, 448, 501–505. [Google Scholar] [CrossRef] [PubMed]
- Chamilos, G.; Gregorio, J.; Meller, S.; Lande, R.; Kontoyiannis, D.P.; Modlin, R.L.; Gilliet, M. Cytosolic sensing of extracellular self-DNA transported into monocytes by the antimicrobial peptide LL37. Blood 2012, 120, 3699–3707. [Google Scholar] [CrossRef] [PubMed]
- Maffei, M.E.; Arimura, G.I.; Mithoefer, A. Natural elicitors, effectors and modulators of plant responses. Nat. Prod. Rep. 2012, 29, 1288–1303. [Google Scholar] [CrossRef] [PubMed]
- Mithoefer, A.; Boland, W. Plant Defense Against Herbivores: Chemical Aspects. Annu. Rev. Plant Biol. 2012, 63, 431–450. [Google Scholar] [CrossRef] [PubMed]
- Nelson, T. The grass leaf developmental gradient as a platform for a systems understanding of the anatomical specialization of C-4 leaves. J. Exp. Bot. 2011, 62, 3039–3048. [Google Scholar] [CrossRef] [PubMed]
- Mohanta, T.K.; Occhipinti, A.; Zebelo, A.S.; Foti, M.; Fliegmann, J.; Bossi, S.; Maffei, M.E.; Bertea, C.M. Ginkgo biloba responds to herbivory by activating early signaling and direct defenses. PLoS ONE 2012, 7, e32822. [Google Scholar] [CrossRef] [PubMed]
- Bricchi, I.; Bertea, C.M.; Occhipinti, A.; Paponov, I.A.; Maffei, M.E. Dynamics of Membrane Potential Variation and Gene Expression Induced by Spodoptera littoralis, Myzus persicae, and Pseudomonas syringae in Arabidopsis. PLoS ONE 2012, 7, e46673. [Google Scholar] [CrossRef] [PubMed]
- Arimura, G.I.; Ozawa, R.; Maffei, M.E. Recent advances in plant early signaling in response to herbivory. Int. J. Mol. Sci. 2011, 12, 3723–3739. [Google Scholar] [CrossRef] [PubMed]
- Williams, S.J.; Sohn, K.H.; Wan, L.; Bernoux, M.; Sarris, P.F.; Segonzac, C.; Ve, T.; Ma, Y.; Saucet, S.B.; Ericsson, D.J.; et al. Structural Basis for Assembly and Function of a Heterodimeric Plant Immune Receptor. Science 2014, 344, 299–303. [Google Scholar] [CrossRef] [PubMed]
- Kagan, J.C. Common mechanisms activate plant guard receptors and TLR4. Trends Immunol. 2014, 35, 454–456. [Google Scholar] [CrossRef] [PubMed]
- Gomez-Gomez, L.; Boller, T. Flagellin perception: A paradigm for innate immunity. Trends Plant Sci. 2002, 7, 251–256. [Google Scholar] [CrossRef]
- Liu, X.G.; Yao, M.; Li, N.; Wang, C.M.; Zheng, Y.Y.; Cao, X. CaMKII promotes TLR-triggered proinflammatory cytokine and type I interferon production by directly binding and activating TAK1 and IRF3 in macrophages. Blood 2008, 112, 4961–4970. [Google Scholar] [CrossRef] [PubMed]
- Kleist, T.J.; Luan, S. Constant change: Dynamic regulation of membrane transport by calcium signalling networks keeps plants in tune with their environment. Plant Cell. Environ. 2016, 39, 467–481. [Google Scholar] [CrossRef] [PubMed]
- Wilke, S. Plant Molecular Biology—A Laboratory Manual; Springer: Berlin, Germany, 1997. [Google Scholar]
- Martinelli, S.; Clark, P.L.; Zucchi, M.I.; Silva-Filho, M.C.; Foster, J.E.; Omoto, C. Genetic structure and molecular variability of Spodoptera frugiperda (Lepidoptera: Noctuidae) collected in maize and cotton fields in Brazil. Bull. Entomol. Res. 2007, 97, 225–231. [Google Scholar] [CrossRef] [PubMed]
- Bricchi, I.; Leitner, M.; Foti, M.; Mithöfer, A.; Boland, W.; Maffei, M.E. Robotic mechanical wounding (MecWorm) versus herbivore-induced responses: Early signaling and volatile emission in Lima bean (Phaseolus lunatus L.). Planta 2010, 232, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Mithöfer, A.; Boland, W.; Maffei, M.E. Chemical ecology of plant insect interactions. In Molecular Aspects of Plant Disease Resistance; Parker, J., Ed.; Wiley-Blackwell: Chirchester, UK, 2009. [Google Scholar]
| Specifications | Cations | ||||
|---|---|---|---|---|---|
| Ammonium | Potassium | Sodium | Calcium | Magnesium | |
| MES buffer | 6.31 (0.91) | 6.15 (0.44) | 39.48 (1.28) | 28.01 (0.16) | 3.08 (0.44) |
| PE buffer | 4.84 (1.45) | 16.33 (0.15) | 2.71 (0.43) | 1.57 (0.08) | 2.71 (0.18) |
| Maize homogenate | 3.41 (0.03) | 1558.28 (21.92) | 58.26 (4.01) | 112.01 (4.08) | 69.63 (4.80) |
| Lima bean homogenate | 1.49 (0.39) | 4307.91 (1.86) | 26.21 (0.99) | 516.21 (10.19) | 208.60 (1.43) |
| S.l. homogenate | 77.34 (9.54) | 3872.34 (291.17) | 554.43 (45.07) | 53.68 (4.88) | 462.29 (32.73) |
| Maize sonicated DNA | 4.88 (0.22) | 15.71 (0.14) | 47.28 (1.39) | 2.83 (0.19) | 2.52 (0.04) |
| Lima bean sonicated DNA | 4.86 (0.30) | 15.86 (0.79) | 124.28 (1.26) | 3.39 (0.27) | 2.28 (0.13) |
| Anova | F17,6 = 86.415 | F17,6 = 11351.986 | F20,6 = 1536.737 | F19,6 = 3758.449 | F18,6 = 1224.789 |
| p values | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 | p < 0.001 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbero, F.; Guglielmotto, M.; Capuzzo, A.; Maffei, M.E. Extracellular Self-DNA (esDNA), but Not Heterologous Plant or Insect DNA (etDNA), Induces Plasma Membrane Depolarization and Calcium Signaling in Lima Bean (Phaseolus lunatus) and Maize (Zea mays). Int. J. Mol. Sci. 2016, 17, 1659. https://doi.org/10.3390/ijms17101659
Barbero F, Guglielmotto M, Capuzzo A, Maffei ME. Extracellular Self-DNA (esDNA), but Not Heterologous Plant or Insect DNA (etDNA), Induces Plasma Membrane Depolarization and Calcium Signaling in Lima Bean (Phaseolus lunatus) and Maize (Zea mays). International Journal of Molecular Sciences. 2016; 17(10):1659. https://doi.org/10.3390/ijms17101659
Chicago/Turabian StyleBarbero, Francesca, Michela Guglielmotto, Andrea Capuzzo, and Massimo E. Maffei. 2016. "Extracellular Self-DNA (esDNA), but Not Heterologous Plant or Insect DNA (etDNA), Induces Plasma Membrane Depolarization and Calcium Signaling in Lima Bean (Phaseolus lunatus) and Maize (Zea mays)" International Journal of Molecular Sciences 17, no. 10: 1659. https://doi.org/10.3390/ijms17101659








