Modified Aloe Polysaccharide Restores Chronic Stress-Induced Immunosuppression in Mice
Abstract
:1. Introduction
2. Results
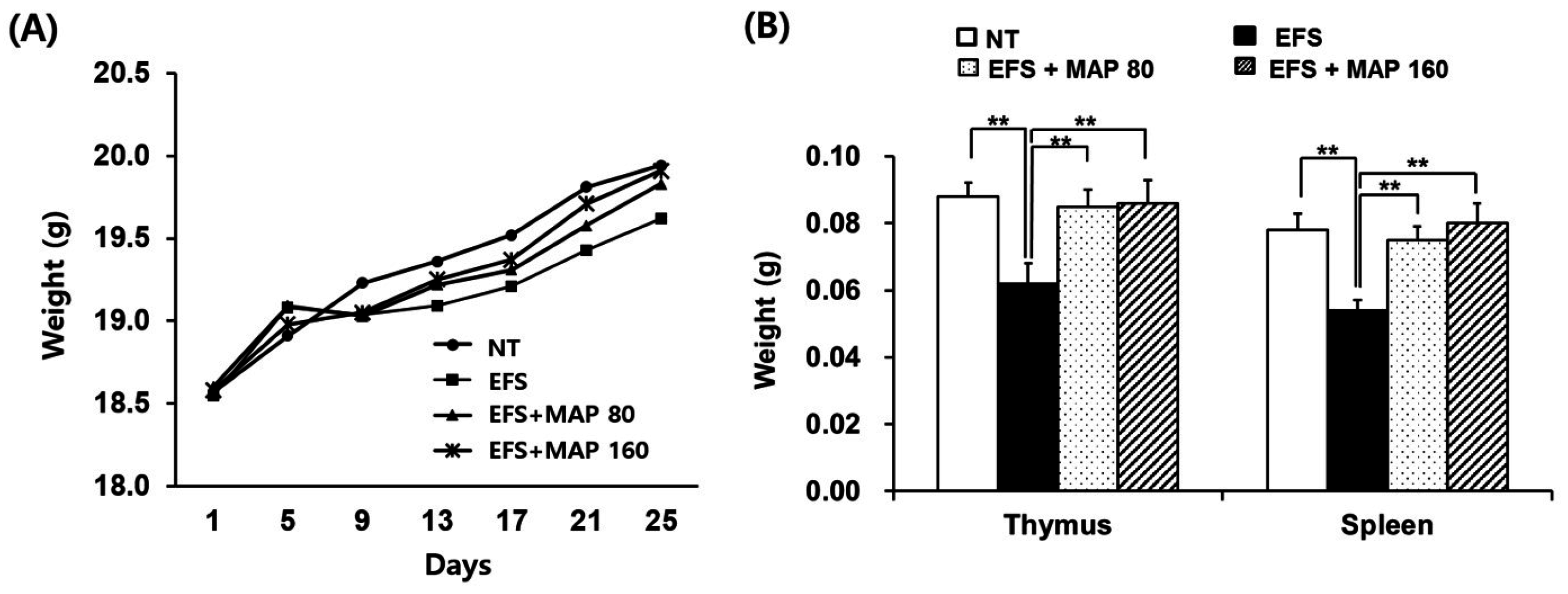
2.1. Alleviation of Chronic Electric Foot Shock (EFS) Stress-Induced Body Weight Loss and Lymphoid Organ Atrophy by Modified Aloe Polysaccharide (MAP)
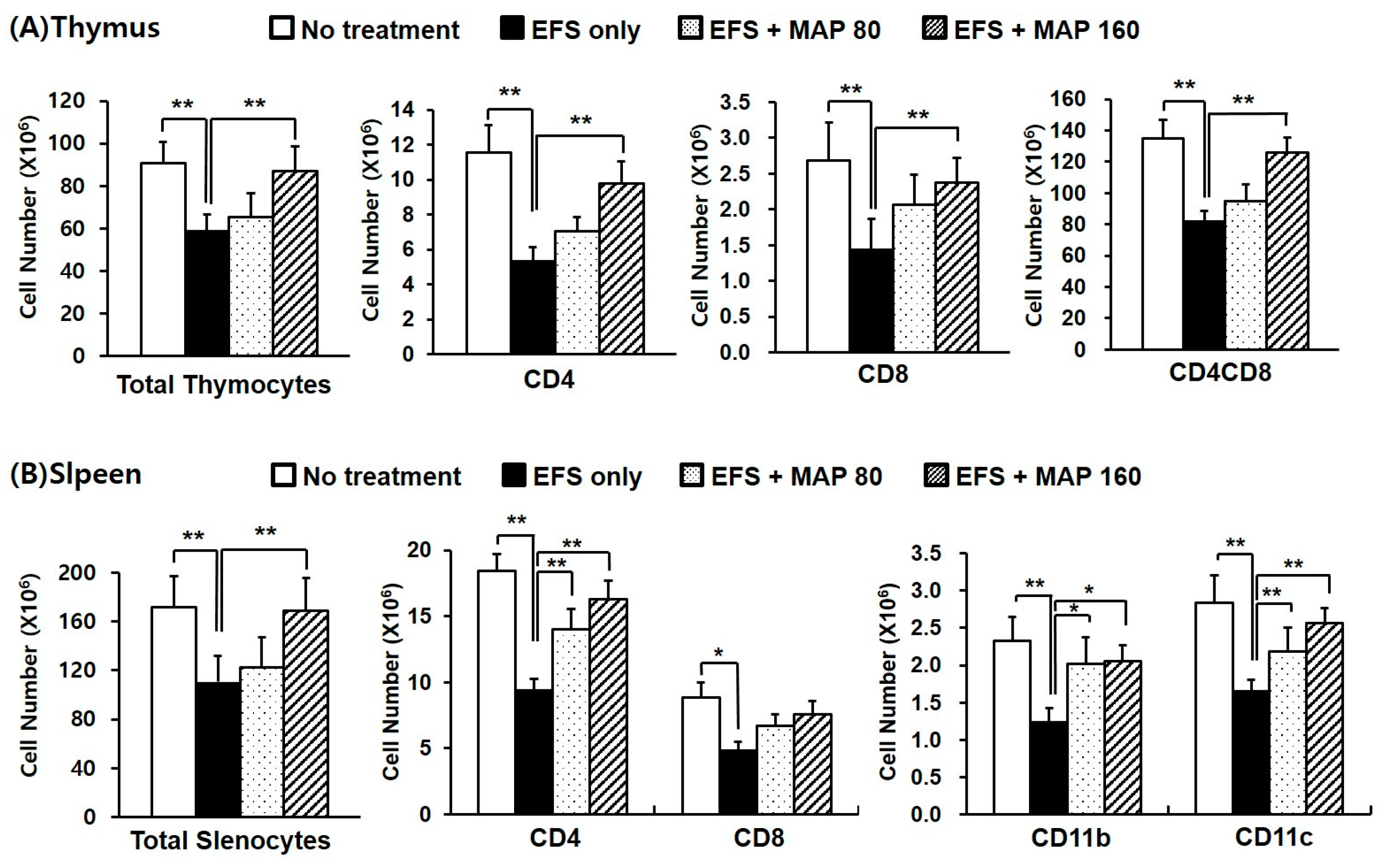
2.2. Alleviative Effect of MAP on Chronic EFS Stress-Induced Disturbances in Lymphocyte Subsets
2.3. Protective Effects of MAP against Chronic EFS Stress-Induced Suppression of Lymphocyte Proliferation
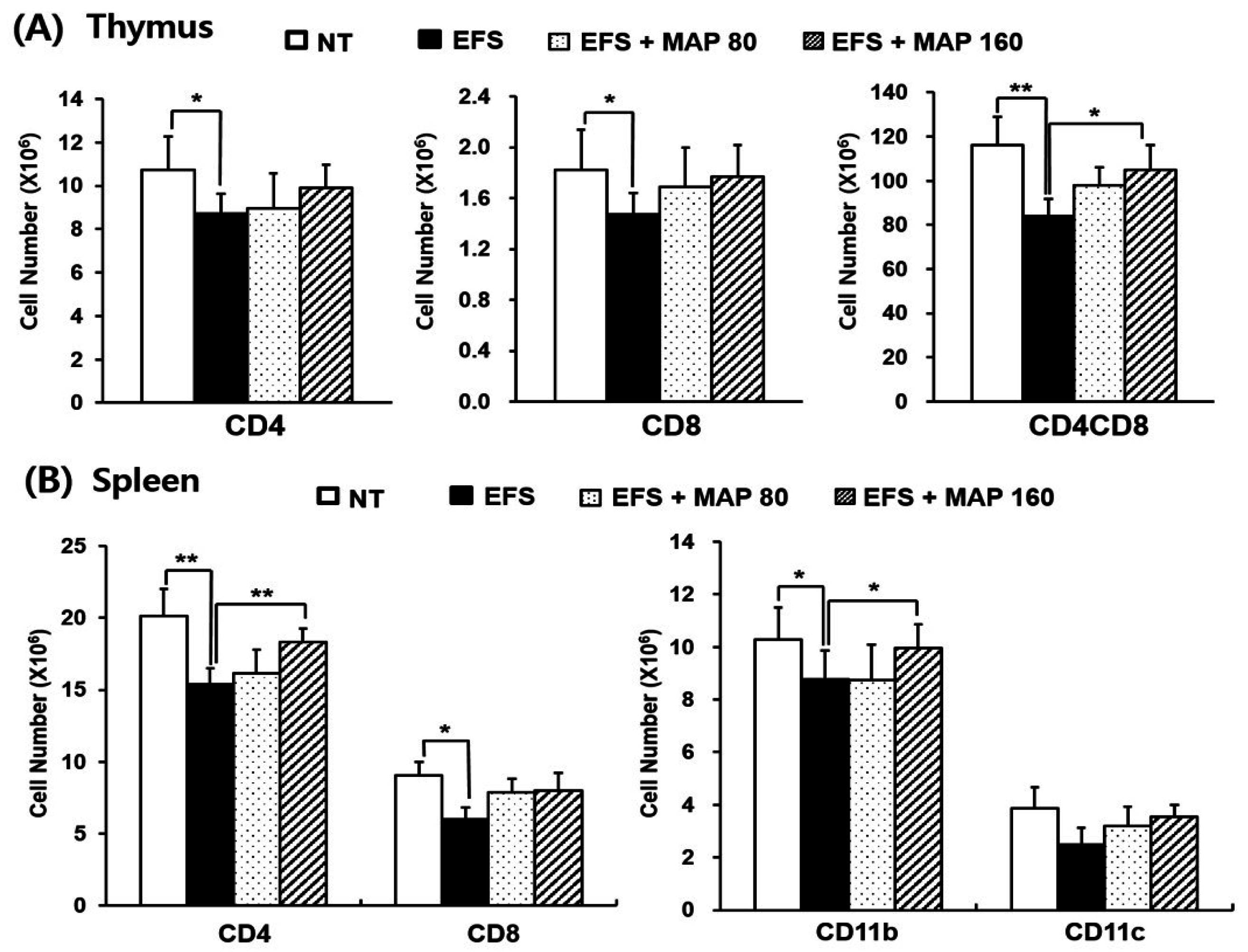
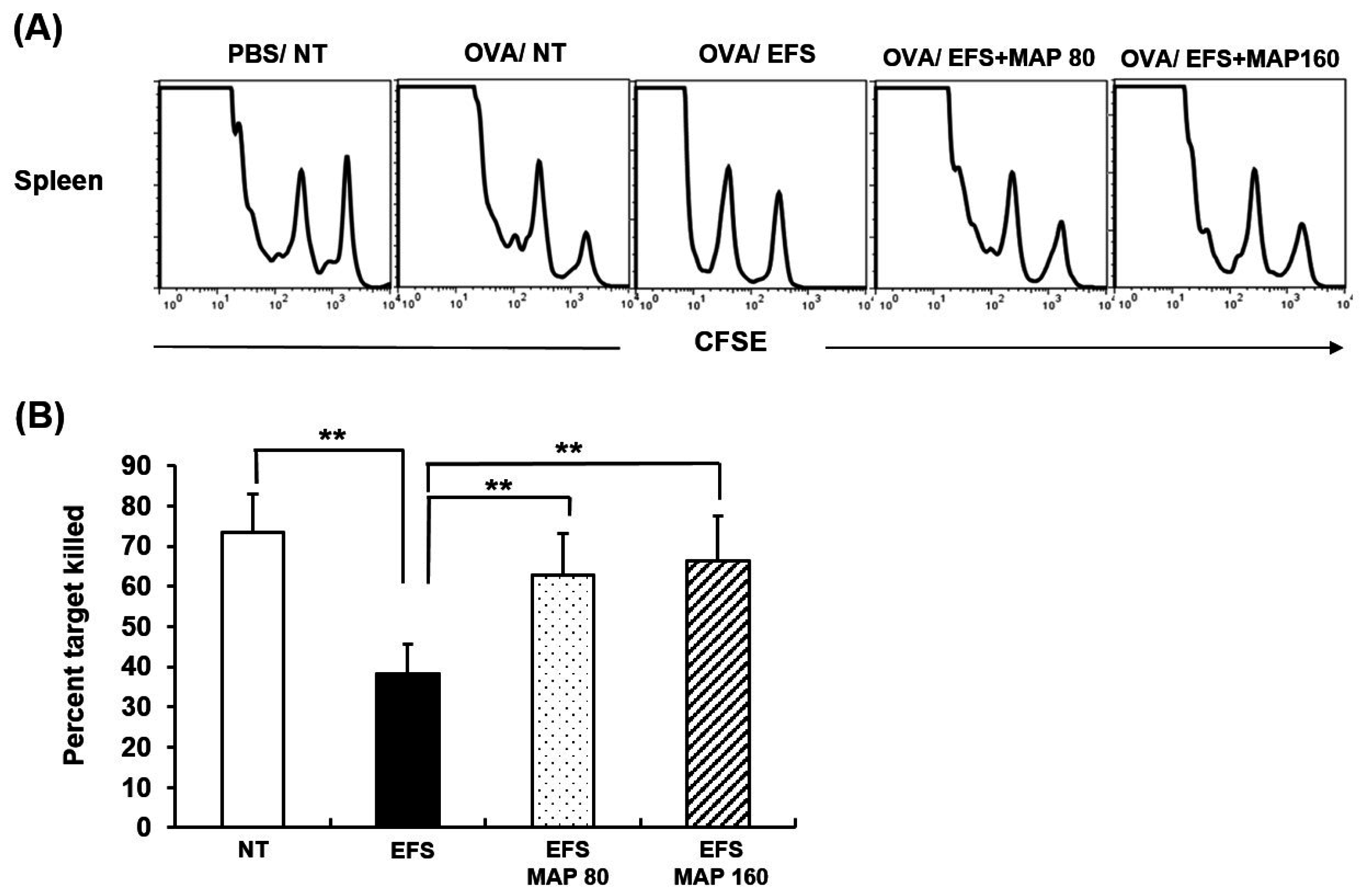
2.4. Protective Effect of MAP against Chronic EFS Stress-Induced Disturbance in Lymphoid Organ Subsets of Ovalbumin (OVA)-Immunized Mice
2.5. Immune Enhancing Effect of MAP on Immunoglobulin G (IgG) Production and Generation of OVA-Specific T Cells in Chronically Stressed Mice
2.6. Immune Enhancing Effect of MAP on Generation of Cytotoxic T Lymphocyte (CTL) in Chronically Stressed Mice
3. Discussion
4. Materials and Methods
4.1. Preparation of MAP
4.2. Animals and Experimental Treatments
4.3. Lymphocyte Subset Analysis
4.4. Lymphocyte Proliferation Assay
4.5. Measurement of Immunoglobulin G (IgG) Concentration in Peripheral Blood
4.6. Proliferation Assay of OVA-Specific T Lymphocytes
4.7. In Vivo Cytotoxic T Lymphocyte (CTL) Assay
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Dhabhar, F.S. Effects of stress on immune function: The good, the bad, and the beautiful. Immunol. Res. 2014, 58, 193–210. [Google Scholar] [CrossRef] [PubMed]
- Segerstrom, S.C.; Miller, G.E. Psychological stress and the human immune system: A meta-analytic study of 30 years of inquiry. Psychol. Bull. 2004, 130, 601–630. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. A hassle a day may keep the doctor away: Stress and the augmentation of immune function. Integr. Comp. Biol. 2002, 42, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Selye, H. A code for coping with stress. Aorn. J. 1977, 25, 35–42. [Google Scholar] [CrossRef]
- Dhabhar, F.S.; Mcewen, B.S. Acute stress enhances while chronic stress suppresses cell-mediated immunity in vivo: A potential role for leukocyte trafficking. Brain Behav. Immun. 1997, 11, 286–306. [Google Scholar] [CrossRef] [PubMed]
- Marshall, G.D., Jr.; Agarwal, S.K.; Lloyd, C.; Cohen, L.; Henninger, E.M.; Morris, G.J. Cytokine dysregulation associated with exam stress in healthy medical students. Brain Behav. Immun. 1998, 12, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Chiappelli, F.; Manfrini, E.; Franceschi, C.; Cossarizza, A.; Black, K.L. Steroid regulation of cytokines: Relevance for TH1-to-TH2 shift? Ann. N. Y. Acad. Sci. 1994, 746, 204–215. [Google Scholar] [CrossRef] [PubMed]
- Balan, B.J.; Niemcewicz, M.; Kocik, J.; Jung, L.; Skopinska-Rozewska, E.; Skopinski, P. Oral administration of Aloe vera gel, anti-microbial and anti-inflammatory herbal remedy, stimulates cell-mediated immunity and antibody production in a mouse model. Cent. Eur. J. Immunol. 2014, 39, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Hart, L.A.; van den Berg, A.J.; Kuis, L.; van Dijk, H.; Labadie, R.P. An anti-complementary polysaccharide with immunological adjuvant activity from the leaf parenchyma gel of Aloe vera. Planta Med. 1989, 55, 509–512. [Google Scholar] [CrossRef]
- Reynolds, T.; Dweck, A.C. Aloe vera leaf gel: A review update. J. Ethnopharmacol. 1999, 68, 3–37. [Google Scholar] [CrossRef]
- Qiu, Z.; Jones, K.; Wylie, M.; Jia, Q.; Orndorff, S. Modified Aloe barbadensis polysaccharide with immunoregulatory activity. Planta Med. 2000, 66, 152–156. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Oh, S.T.; Song, S.; Kim, M.R.; Kim, D.S.; Woo, S.S.; Jo, T.H.; Park, Y.I.; Lee, C.K. Identification of optimal molecular size of modified Aloe polysaccharides with maximum immunomodulatory activity. Int. Immunopharmacol. 2005, 5, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Lysle, D.T.; Lyte, M.; Fowler, H.; Rabin, B.S. Shock-induced modulation of lymphocyte reactivity: Suppression, habituation, and recovery. Life Sci. 1987, 41, 1805–1814. [Google Scholar] [CrossRef]
- Batuman, O.A.; Sajewski, D.; Ottenweller, J.E.; Pitman, D.L.; Natelson, B.H. Effects of repeated stress on T cell numbers and function in rats. Brain Behav. Immun. 1990, 4, 105–117. [Google Scholar] [CrossRef]
- Zhao, T.T.; Shin, K.S.; Choi, H.S.; Lee, M.K. Ameliorating effects of gypenosides on chronic stress-induced anxiety disorders in mice. BMC Complement. Altern. Med. 2015. [Google Scholar] [CrossRef] [PubMed]
- Choi, H.S.; Park, M.S.; Kim, S.H.; Hwang, B.Y.; Lee, C.K.; Lee, M.K. Neuroprotective effects of herbal ethanol extracts from Gynostemma pentaphyllum in the 6-hydroxydopamine-lesioned rat model of Parkinson’s disease. Molecules 2010, 15, 2814–2824. [Google Scholar] [CrossRef] [PubMed]
- Sutanto, W.; de Kloet, E.R. The use of various animal models in the study of stress and stress-related phenomena. Lab. Anim. 1994, 28, 293–306. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, N.; Maiti, P.P.; Choudhary, A.; Tuli, A.; Masih, D.; Masih, M.; Khan, U.; Ara, T.; Jaggi, A.S. Animal models in the study of stress: A review. NSHM J. Pharm. Healthc. Manag. 2011, 2, 42–50. [Google Scholar]
- Im, S.A.; Choi, H.S.; Choi, S.O.; Kim, K.H.; Lee, S.; Hwang, B.Y.; Lee, M.K.; Lee, C.K. Restoration of electric footshock-induced immunosuppression in mice by Gynostemma pentaphyllum components. Molecules 2012, 17, 7695–7708. [Google Scholar] [CrossRef] [PubMed]
- McEwen, B.S. Protective and damaging effects of stress mediators: The good and bad sides of the response to stress. Metabolism 2002, 51, 2–4. [Google Scholar] [CrossRef] [PubMed]
- Ben-Eliyahu, S.; Yirmiya, R.; Liebeskind, J.C.; Taylor, A.N.; Gale, R.P. Stress increases metastatic spread of a mammary tumor in rats: Evidence for mediation by the immune system. Brain Behav. Immun. 1991, 5, 193–205. [Google Scholar] [CrossRef]
- Black, P.H.; Garbutt, L.D. Stress, inflammation and cardiovascular disease. J. Psychosom. Res. 2002, 52, 1–23. [Google Scholar] [CrossRef]
- Dhabhar, F.S. Enhancing versus suppressive effects of stress on immune function: implications for immunoprotection and immunopathology. Neuroimmunomodulation 2009, 16, 300–317. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S.; Malarkey, W.B.; Neri, E.; McEwen, B.S. Stress-induced redistribution of immune cells—From barracks to boulevards to battlefields: A tale of three hormones—Curt Richter Award winner. Psychoneuroendocrinology 2012, 37, 1345–1368. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.X.; Denhardt, D.T. Osteopontin: Role in immune regulation and stress responses. Cytokine Growth Factor Rev. 2008, 19, 333–345. [Google Scholar] [CrossRef] [PubMed]
- Parham, P. The Immune System, 4th ed.; Garland Science: New York, NY, USA, 2005; pp. 218–226. [Google Scholar]
- Purton, J.F.; Monk, J.A.; Liddicoat, D.R.; Kyparissoudis, K.; Sakkal, S.; Richardson, S.J.; Godfrey, D.I.; Cole, T.J. Expression of the glucocorticoid receptor from the 1A promoter correlates with T lymphocyte sensitivity to glucocorticoid-induced cell death. J. Immunol. 2004, 173, 3816–3824. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.H.; Lee, Y.R.; Im, S.A.; Park, S.I.; Kim, K.H.; Gerelchuluun, T.; Song, S.; Kim, K.; Lee, C.K. Calcineurin inhibitors block MHC-restricted antigen presentation in vivo. J. Immunol. 2007, 179, 5711–5716. [Google Scholar] [CrossRef] [PubMed]
- Im, S.A.; Lee, Y.R.; Lee, Y.H.; Lee, M.K.; Park, Y.I.; Lee, S.; Kim, K.; Lee, C.K. In vivo evidence of the immunomodulatory activity of orally administered Aloe vera gel. Arch. Pharm. Res. 2010, 33, 451–456. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, Y.; Im, S.-A.; Kim, J.; Lee, S.; Kwon, J.; Lee, H.; Kong, H.; Song, Y.; Shin, E.; Do, S.-G.; et al. Modified Aloe Polysaccharide Restores Chronic Stress-Induced Immunosuppression in Mice. Int. J. Mol. Sci. 2016, 17, 1660. https://doi.org/10.3390/ijms17101660
Lee Y, Im S-A, Kim J, Lee S, Kwon J, Lee H, Kong H, Song Y, Shin E, Do S-G, et al. Modified Aloe Polysaccharide Restores Chronic Stress-Induced Immunosuppression in Mice. International Journal of Molecular Sciences. 2016; 17(10):1660. https://doi.org/10.3390/ijms17101660
Chicago/Turabian StyleLee, Youngjoo, Sun-A Im, Jiyeon Kim, Sungwon Lee, Junghak Kwon, Heetae Lee, Hyunseok Kong, Youngcheon Song, Eunju Shin, Seon-Gil Do, and et al. 2016. "Modified Aloe Polysaccharide Restores Chronic Stress-Induced Immunosuppression in Mice" International Journal of Molecular Sciences 17, no. 10: 1660. https://doi.org/10.3390/ijms17101660
APA StyleLee, Y., Im, S. -A., Kim, J., Lee, S., Kwon, J., Lee, H., Kong, H., Song, Y., Shin, E., Do, S. -G., Lee, C. -K., & Kim, K. (2016). Modified Aloe Polysaccharide Restores Chronic Stress-Induced Immunosuppression in Mice. International Journal of Molecular Sciences, 17(10), 1660. https://doi.org/10.3390/ijms17101660