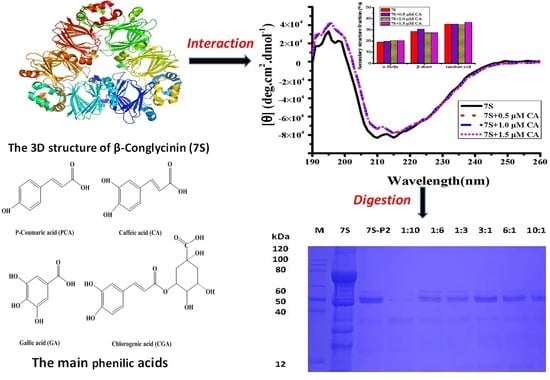
Interactions of β-Conglycinin (7S) with Different Phenolic Acids—Impact on Structural Characteristics and Proteolytic Degradation of Proteins
Abstract
:1. Introduction
2. Results and Discussion
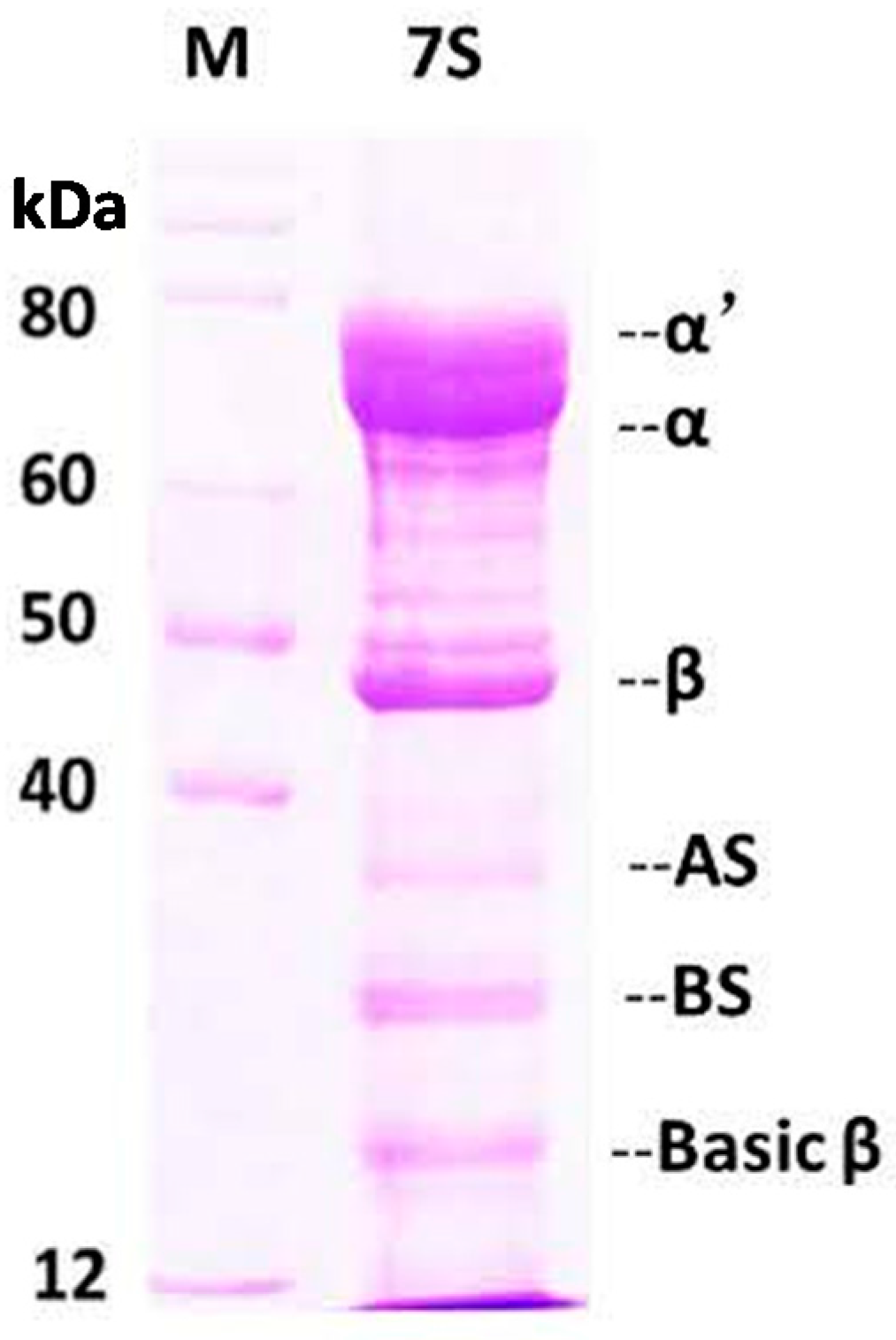
2.1. Purification and Characterization of Soy Protein 7S
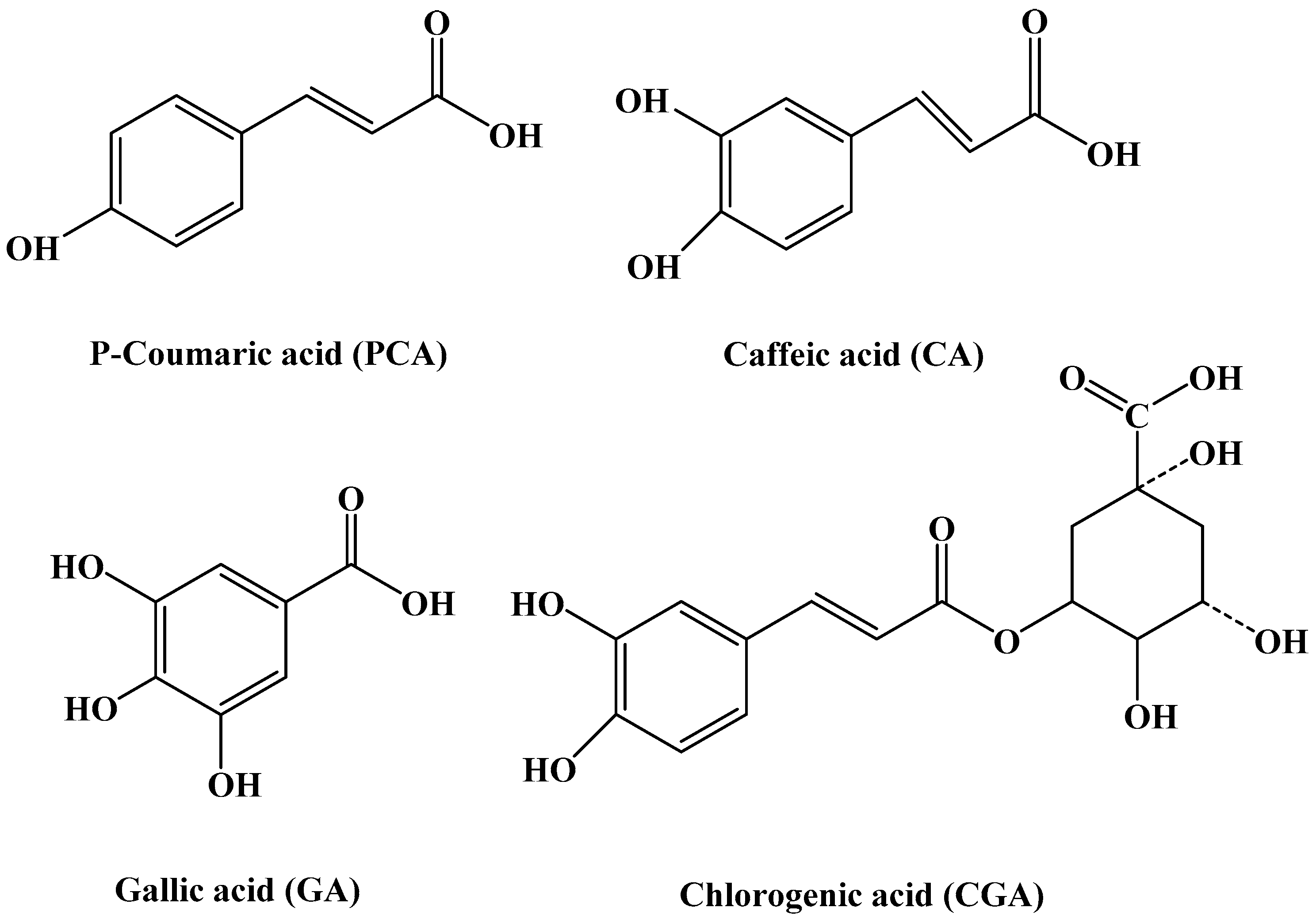
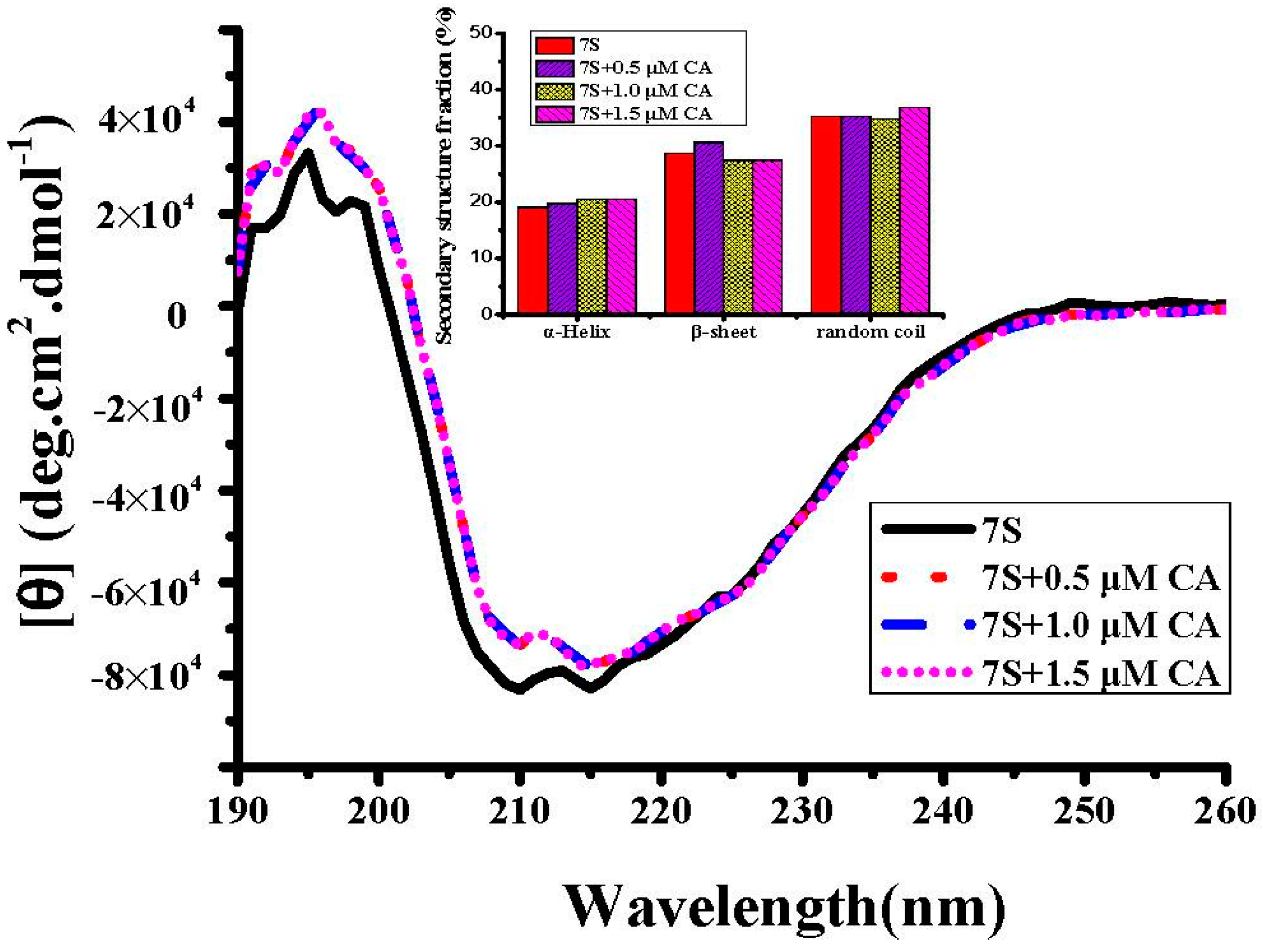
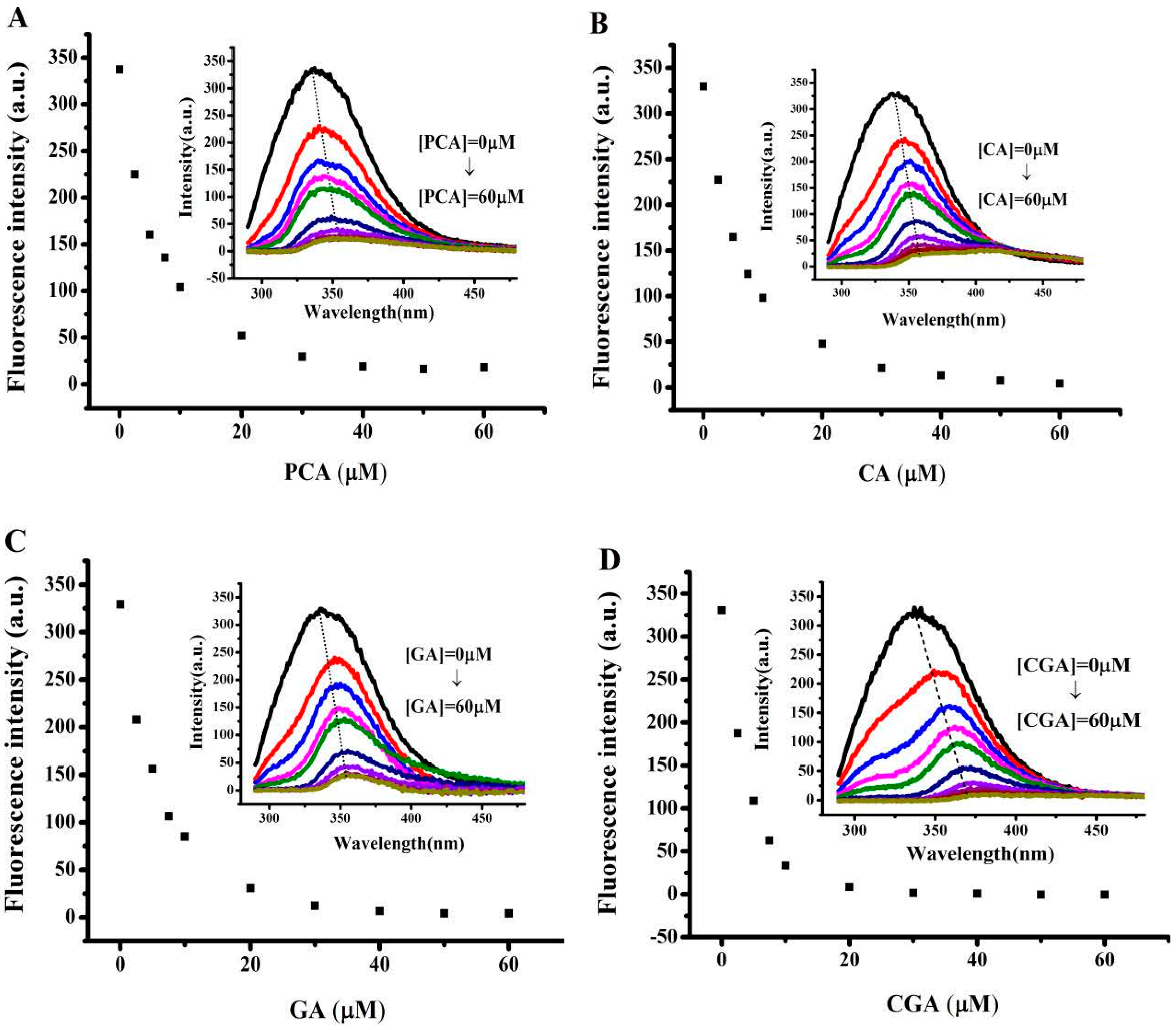
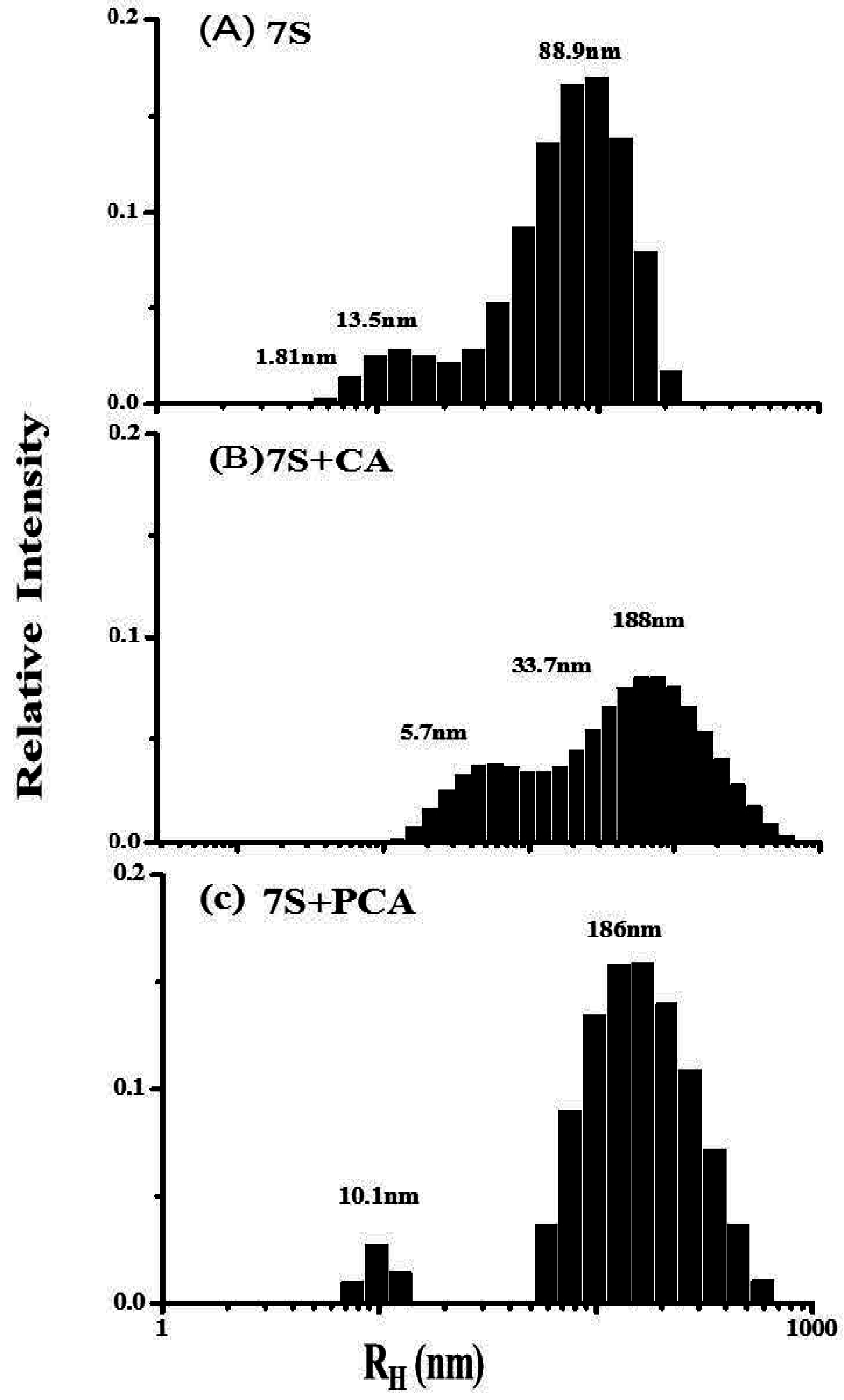
2.2. Interaction Between Different Phenolic Acids and Soy Protein 7S
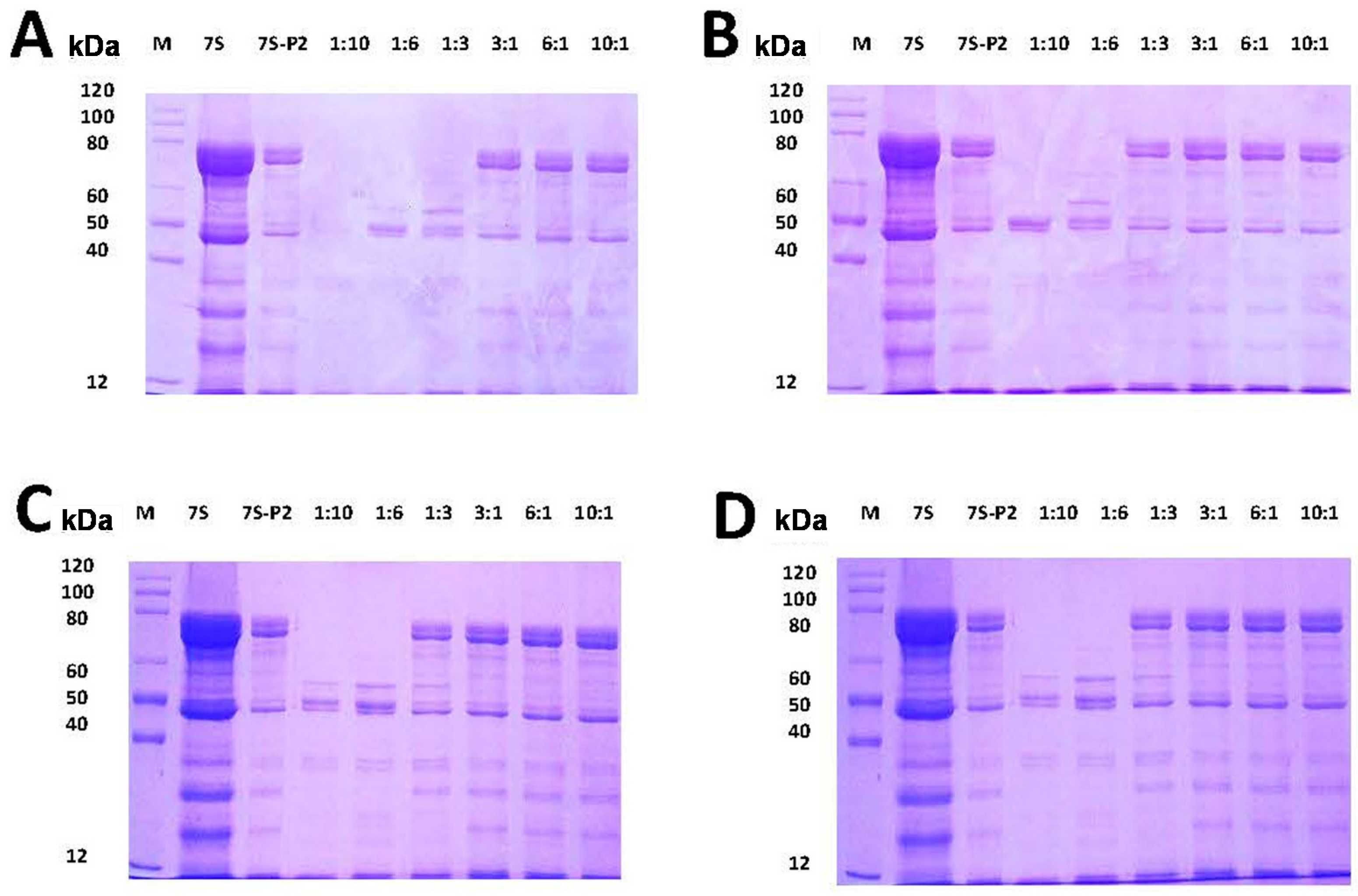
2.3. Effect of Different Phenolic Acids on the Digestive Stability of Soy Protein 7S
3. Materials and Methods
3.1. Chemicals
3.2. Preparation of Soy Protein 7S
3.3. Fluorescence Titration
3.4. Circular Dichroism (CD) Spectra
3.5. Dynamic Light-Scattering (DLS) Experiments
3.6. Simulated Gastric Fluid and Simulated Intestinal Fluid Digestion Stability Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| PCA | p-Coumalic Acid |
| CA | Caffeic |
| GA | Gallic Acid |
| CGA | Chlorogenic Acid |
| 7S | β-Conglycinin |
| CD | Circular Dichroism |
| DLS | Dynamic Light-Scattering |
References
- Moriyama, T.; Kishimoto, K.; Nagai, K.; Urade, R.; Ogawa, T.; Utsumi, S.; Maruyama, N.; Maebuchi, M. Soybean β-conglycinin diet suppresses serum triglyceride levels in normal and genetically obese mice by induction of β-oxidation, downregulation of fatty acid synthase, and inhibition of triglyceride absorption. Biosci. Biotechnol. Biochem. 2004, 68, 352–359. [Google Scholar] [CrossRef] [PubMed]
- Yamauchi, F.; Thanh, V.H.; Kawase, M.; Kazuo, S. Separation of the Glycopeptides from Soybean 7S Protein: Their Amino Acid Sequences. Agric. Biol. Chem. 1976, 40, 691–696. [Google Scholar]
- Nobuyuki, M.; Takako, F.; Shiori, S.; Nauko, I.; Junko, K.; Mayumi, M.; Misa, H.; Machiko, S.; Tatsuya, M.; Shigeru, U. Molecular and structural analysis of electrophoretic variants of soybean seed storage proteins. Phytochemistry 2003, 64, 701–708. [Google Scholar]
- Cruz, N.S.; Capellas, M.; Jaramillo, D.P.; Trujillo, A.J.; Guamis, B.; Ferragut, V. Soymilk treated by ultra-high-pressure homogenization: acid coagulation properties and characteristics of a soy-yogurt product. Food Hydrocoll. 2009, 23, 490–496. [Google Scholar] [CrossRef]
- Tang, C.H.; Luo, L.J.; Liu, F.U.; Chen, Z. Transglutaminase-set soy globulin-stabilized emulsion gels: Influence of soy β-conglycinin/glycinin ratio on properties, microstructure and gelling mechanism. Food Res. Int. 2013, 51, 804–812. [Google Scholar] [CrossRef]
- Scarafoni, A.; Magni, C.; Duranti, M. Molecular nutraceutics as a mean to investigate the positive effects of legume seed proteins on human healthy. Trends Food Sci. Technol. 2007, 18, 454–463. [Google Scholar] [CrossRef]
- Lovati, M.R.; Manzoni, C.; Corsini, A.; Granata, A.; Fumagalli, R.; Sirtori, C.R. Low density lipoprotein receptor activity is modulated by soybean globulins in cell culture. J. Nutr. 1992, 122, 1971–1978. [Google Scholar]
- Marcello, D.; Maria Rosa, L.; Valeria, D.; Alberto, B.; Alessio, S.; Silvia, C. The α’ subunit from soybean 7S globulin lowers plasma lipids and upregulates liver β-VLDL receptors in rats fed a hypercholesterolemic diet. J. Nutr. 2004, 134, 1334–1339. [Google Scholar]
- Molina, E.; Papadopoulou, A.; Ledward, D.A. Emulsifying properties of high pressure treated soy protein isolate and 7S and 11S globulins. Food Hydrocoll. 2001, 15, 263–269. [Google Scholar] [CrossRef]
- Peng, X.; Guo, S. Texture characteristics of soymilk gels formed by lactic fermentation: A comparison of soymilk prepared by blanching soybeans under different temperatures. Food Hydrocoll. 2015, 43, 58–65. [Google Scholar] [CrossRef]
- Li, J.; Li, Y.; Guo, S. The binding mechanism of lecithin to soybean 11S and 7S globulins using fluorescence spectroscopy. Food Sci. Biotechnol. 2014, 23, 1785–1791. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Impact of high intensity pulsed electric field on antioxidant properties and quality parameters of a fruit juice-soymilk beverage in chilled storage. LWT-Food Sci. Technol. 2010, 43, 872–881. [Google Scholar] [CrossRef]
- Morales-de la Peña, M.; Salvia-Trujillo, L.; Rojas-Graü, M.A.; Martín-Belloso, O. Changes on phenolic and carotenoid composition of high intensity pulsed electric field and thermally treated fruit juice-soymilk beverages during refrigerated storage. Food Chem. 2011, 129, 982–990. [Google Scholar] [CrossRef] [PubMed]
- Morales-de la Peña, M.; Salvia-Trujillo, L.; Garde-Cerdan, T.; Rojas-Graü, M.A.; Martín-Belloso, O. High intensity pulsed electric fields or thermal treatments effects on the amino acid profile of a fruit juice-soymilk beverage during refrigeration storage. Innov. Food Sci. Emerg. Technol. 2012, 16, 47–53. [Google Scholar] [CrossRef]
- Potter, R.M.; Dougherty, M.P.; Halteman, W.A.; Camire, M.E. Characteristics of wild blueberry-soy beverages. LWT-Food Sci. Technol. 2007, 40, 807–814. [Google Scholar] [CrossRef]
- Ribnicky, D.M.; Roopchand, D.E.; Poulev, A.; Kuhn, P.; Oren, A.; Cefalu, W.T.; Raskin, I. Artemisia dracunculus L. polyphenols complexed to soy protein show enhanced bioavailability and hypoglycemic activity in C57BL/6 mice. Nutrition 2014, 30, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Stojadinovic, M.; Radosavljevic, J.; Ognjenovic, J.; Vesic, J.; Prodic, I.; Stanic-Vucinic, D.; Velickovic, C.T. Binding affinity between dietary polyphenols and β-lactoglobulin negatively correlates with the protein susceptibility to digestion and total antioxidant activity of complexes formed. Food Chem. 2013, 136, 1263–1271. [Google Scholar] [CrossRef] [PubMed]
- Labuckas, D.O.; Maestri, D.M.; Perelló, M.; Martínez, M.L.; Lamarque, A.L. Phenolics from walnut (Juglans regia L.) kernels: Antioxidant activity and interactions with proteins. Food Chem. 2008, 107, 607–612. [Google Scholar] [CrossRef]
- Rawel, H.M.; Czajka, D.; Rohn, S.; Kroll, J. Interactions of different phenolic acids and flavonoids with soy proteins. Int. J. Biol. Macromol. 2002, 30, 137–150. [Google Scholar] [CrossRef]
- Rawel, H.M.; Kroll, J.; Rohn, S. Reactions of phenolic substances with lysozyme-physicochemical characterisation and proteolytic digestion of the derivatives. Food Chem. 2001, 72, 59–71. [Google Scholar] [CrossRef]
- Petzke, K.J.; Stefanie, S.; Sascha, R.; Rawel, H.M.; Jürgen, K. Chlorogenic acid moderately decreases the quality of whey proteins in rats. J. Agric. Food Chem. 2005, 53, 3714–3720. [Google Scholar] [CrossRef] [PubMed]
- Rohn, S.; Petzke, K.J.; Rawel, H.M.; Kroll, J. Reactions of chlorogenic acid and quercetin with a soy protein isolate—Influence on the in vivo food protein quality in rats. Mol. Nutr. Food Res. 2006, 50, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Bellion, P.; Dioles, J.; Will, F. Polyphenolic apple extracts: Effects of raw material and production method on antioxidant effectiveness and reduction of DNA damage in Caco-2 cells. J. Agric. Food Chem. 2010, 58, 6636–6642. [Google Scholar] [CrossRef] [PubMed]
- Bomser, J.; Madhavi, D.L.; Singletary, K.; Smith, M.A.L. In vitro anticancer activity of fruit extracts from Vaccinium species. Planta Med. 1996, 62, 212–216. [Google Scholar] [CrossRef] [PubMed]
- Crozier, A.; Jaganath, I.B.; Clifford, M.N. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat. Prod. Rep. 2009, 26, 1001–1043. [Google Scholar] [CrossRef] [PubMed]
- Landbo, A.K.; Meyer, A.S. Enzyme-assisted extraction of antioxidative phenols from black currant juice residue (Ribesnigrum). J. Agric. Food Chem. 2001, 49, 3169–3177. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Moghadasian, M.H. Chemistry, natural sources, dietary intake and pharmacokinetic properties of ferulic acid: A review. Food Chem. 2008, 109, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Phoon, P.Y.; Narsimhan, G. Effect of hydrolysis of soy β-conglycinin on the oxidative stability of o/wemulsions. Food Hydrocoll. 2014, 35, 429–443. [Google Scholar] [CrossRef]
- Li, M.; Jia, X.; Yang, J. Effect of tannic acid on properties of soybean (Glycine maxseed ferritin: A model for interaction between naturally-occurring components in foodstuffs. Food Chem. 2012, 133, 410–415. [Google Scholar] [CrossRef] [PubMed]
- Deng, J.; Liao, X.; Yang, H.; Zhang, X.; Hua, Z.; Masuda, T.; Goto, F.; Yoshihara, T.; Zhao, G. Role of H-1 and H-2 subunits of soybean seed ferritin in oxidative deposition of iron in protein. J. Biol. Chem. 2010, 285, 32075–32086. [Google Scholar] [CrossRef] [PubMed]
- Li, C.R.; Fu, X.P.; Xin, Q.; Hu, X.S.; Chasteen, N.D.; Zhao, G.H. Protein association and dissociation regulated by ferric ion: A novel pathway for oxidative deposition of iron in pea seed ferritin. J. Biol. Chem. 2009, 284, 16743–16751. [Google Scholar] [CrossRef] [PubMed]
- Akkermans, C.; Van der Goot, A.J.; Venema, P.; Gruppen, H.; Vereijken, J.M.; van der Linden, E.; Boom, R.M. Micrometer-sized fibrillar protein aggregates from soy glycinin and soy protein isolate. J. Agric. Food Chem. 2007, 55, 9877–9882. [Google Scholar] [CrossRef] [PubMed]
- Argos, P.; Narayana, S.V.L.; Nielsen, N.C. Structural similarity between legumin and vicilin storage proteins from legumes. EMBO J. 1985, 4, 1111–1117. [Google Scholar] [PubMed]
- Callis, P.R. 1Ia and 1Ib transitions of tryptophan: Applications of theory and experimental observations to fluorescence of proteins. Methods Enzymol. 1997, 278, 113–150. [Google Scholar] [PubMed]
- Hasni, I.; Bourassa, P.; Hamdani, S.; Samson, G.; Carpentier, R.; Tajmir-Riahi, H.A. Interaction of milk α- and β-casein with tea polyphenols. Food Chem. 2011, 126, 630–639. [Google Scholar] [CrossRef]
- Kanakis, C.D.; Hasni, I.; Bourassa, P.; Tarantilis, P.A.; Polissiou, M.G. Milk β-lactoglobulin complexes with tea polyphenols. Food Chem. 2011, 127, 1046–1055. [Google Scholar] [CrossRef] [PubMed]
- Kroll, J.; Rawel, H.M.; Rohn, S.; Czajka, D. Interactions of glycinin with plant phenols-influence on chemical properties and proteolytic degradation of the proteins. Mol. Nutr. Food Res. 2001, 45, 388–389. [Google Scholar] [CrossRef]
- Mateus, C.; Carvalho, E.; Luis, C.; de Freitas, V. Influence of the tannin structur e on the disruption effect of carbohydrates on protein-tannin aggregates. Anal. Chim. Acta 2004, 513, 135–140. [Google Scholar] [CrossRef]
- Yuksel, Z.; Avcı, E.; Erdem, Y.K. Characterization of binding interactions between green tea flavanoids and milk proteins. Food Chem. 2010, 121, 450–456. [Google Scholar] [CrossRef]
- Ojha, H.; Mishra, K.; Hassan, M.I.; Chaudhury, N.K. Spectroscopic and isothermal titration calorimetry studies of binding interaction of ferulic acid with bovine serum albumin. Thermochim. Acta 2012, 548, 56–64. [Google Scholar] [CrossRef]
- Duodu, K.G.; Taylor, J.R.N.; Belton, P.S.; Hamaker, B.R. Factors affecting sorghum protein digestibility. J. Cereal Sci. 2003, 38, 117–131. [Google Scholar] [CrossRef] [Green Version]
- Nagano, T.; Hirotsuka, M.; Mori, H.; Kohyama, K.; Nishinari, K. Dynamic viscoelastic study on the gelation of 7 S globulin from soybeans. J. Agric. Food Chem. 1992, 40, 941–944. [Google Scholar] [CrossRef]
- Anonymous Simulated gastric fluid and simulated intestinal fluid, TS. The United States Pharmacopeia 23, the National Formulary 18; The United States Pharmacopeial Convention, Inc.: Rockville, MD, USA, 1995; p. 2053. [Google Scholar]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, J.; Chen, H.; Liu, J.; Wang, Y.; Nirasawa, S.; Cheng, Y. Interactions of β-Conglycinin (7S) with Different Phenolic Acids—Impact on Structural Characteristics and Proteolytic Degradation of Proteins. Int. J. Mol. Sci. 2016, 17, 1671. https://doi.org/10.3390/ijms17101671
Gan J, Chen H, Liu J, Wang Y, Nirasawa S, Cheng Y. Interactions of β-Conglycinin (7S) with Different Phenolic Acids—Impact on Structural Characteristics and Proteolytic Degradation of Proteins. International Journal of Molecular Sciences. 2016; 17(10):1671. https://doi.org/10.3390/ijms17101671
Chicago/Turabian StyleGan, Jing, Hao Chen, Jiyuan Liu, Yongquan Wang, Satoru Nirasawa, and Yongqiang Cheng. 2016. "Interactions of β-Conglycinin (7S) with Different Phenolic Acids—Impact on Structural Characteristics and Proteolytic Degradation of Proteins" International Journal of Molecular Sciences 17, no. 10: 1671. https://doi.org/10.3390/ijms17101671
APA StyleGan, J., Chen, H., Liu, J., Wang, Y., Nirasawa, S., & Cheng, Y. (2016). Interactions of β-Conglycinin (7S) with Different Phenolic Acids—Impact on Structural Characteristics and Proteolytic Degradation of Proteins. International Journal of Molecular Sciences, 17(10), 1671. https://doi.org/10.3390/ijms17101671