New Insights into Various Production Characteristics of Streptococcus thermophilus Strains
Abstract
:1. Introduction
2. Diversity of Genomes and Plasmids of S. thermophilus
2.1. Diversity of S. thermophilus Genomes
2.2. Plasmid Diversity of S. thermophilus
3. Technological and Functional Properties of S. thermophilus
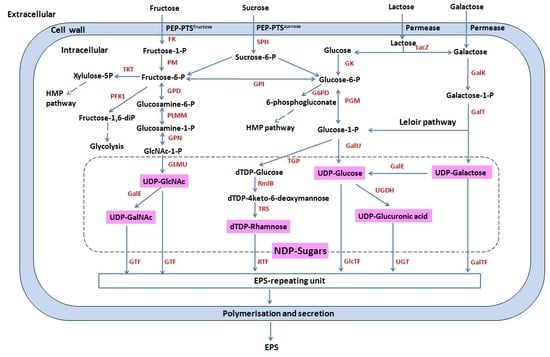
3.1. Sugar Metabolism
3.2. Polysaccharide Biosynthesis
3.3. Proteolytic System and Amino Acid Metabolism
3.4. Flavor
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AlcDH | Alcohol dehydrogenase |
| BcAT | Branched-chain aminotransferase |
| CPSs | Capsular polysaccharides |
| EPSs | Exopolysaccharides |
| EstA | Esterase A |
| GDH | Glutamate dehydrogenase |
| GTFs | Glycosyltransferases |
| IS | Insertion sequence |
| LAB | Lactic acid bacteria |
| l-HycDH | l-hydroxyacid dehydrogenase |
| PTA | Phosphotransacylase |
| RCR | Rolling circle replication |
Appendix A
| Plasmid Name | Strain | Replication | Size (kb) | (G+C)% | Protein | Gene | Pseudogene | Rep Protein | Mob Protein | Function | NCBI Accession | Ref. | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| pK1002C2 | K1002C2 | 3.38 | 35 | 2 | 2 | - | RepA 314 aa | Small heat shock protein | NC_019231.1 | [12] | |||
| pK2007C6 | K2007C6 | 2.98 | 35.1 | 2 | 2 | - | RepA 314 aa | Small heat shock protein | NC_019232.1 | [12] | |||
| pSTER_A | LMD-9 | RCR | 4.45 | 37 | 4 | 4 | - | DNA segregation ATPase FtsK/SpoIIIE or related protein | NC_008500.1 | [25] | Yogurt | ||
| pSTER_B | LMD-9 | RCR | 3.36 | 35.1 | 2 | 2 | - | Rep 314 aa | Small heat shock protein | NC_008501.1 | [25] | Yogurt | |
| pER341 | ST134 | RCR | 2.798 | 33.7 | 2 | AF019139.1 | [31] | An in-house culture collection | |||||
| pCI65st | NDI-6 | RCR | 6.5 | 34.5 | 5 | 5 | RepA 315 aa | Small heat shock protein; enolase | AF027167.1 | [32] | Indian fermented milk dahi | ||
| pND103 | ST2-1 | 3.53 | 32.4 | 4 | 4 | - | NC_004747.1 | [33] | |||||
| pSt0 | St0 | RCR | 8.1 | 37 | 6 | 6 | Cytosine-specific methyltransferase; type II restriction endonuclease | NC_025154 | [34] | Dairy product | |||
| pSt04 | St04 | RCR | 3.1 | Small heat shock protein | AJ242477 | [34] | Dairy product | ||||||
| pSt08 | St08 | RCR | 7.51 | 9 | 1 | Rep 313 aa | Putative adenine specific methyl transferase | AJ 239049 | [34] | Dairy product | |||
| pSt106 | 5.283 | 36 | 1 | Rep 287 aa | Putative resolvase | AJ 242479 | [34] | Dairy product | |||||
| pJ34 | J34 | RCR | 3.38 | 1 | RepA 315 aa | AJ242475 | [34] | Dairy product | |||||
| pSt22-2 | St22 | [34] | Dairy product | ||||||||||
| pER1-1 | RCR | 3.365 | 2 | 1 | RepA 314 aa | Small heat shock protein | AJ 242476 | [34] | Dairy product | ||||
| pER1-2 | 4.45 | 36.9 | 5 | 5 | NC_025196.1 | [34] | Dairy product | ||||||
| pt38 | ST2783 | 2.91 | 32.4 | 5 | 9 | - | Rep 311 aa | NC_005098.1 | [35] | Bulgarian yogurt | |||
| pER16 | 4.27 | 3 | Rep 315 aa | Small heat shock protein | AF177166 | [37] | |||||||
| pER35 | ST135 | 9.53 | 36.5 | 5 | 5 | - | RepA 315 aa | Small heat shock protein; type IC restriction subunit; type IC modification subunit; type IC modification subunit | NC_000937.1 | [37] | |||
| pER36 | ST136 | 3.5 | 34.4 | 2 | 2 | - | RepA 315 aa | Small heat shock protein | NC_000938.1 | [37] | |||
| pSMQ172 | SMQ-172 | RCR | 4.23 | 38 | 4 | 5 | - | Rep 223 aa | Mob 499 aa | NC_004958.1 | [38] | ||
| pSMQ-316 | θ | 6.71 | 37.7 | 5 | 5 | - | Primase-helicase; integrase | NC_010859.1 | [39] | ||||
| pSMQ-312b | θ | [39] | |||||||||||
| pSMQ173b | SMQ-173 | RCR | 4.45 | 37 | 5 | 5 | - | Rep 146 aa | NC_005323.1 | [102] | |||
| pSMQ-308 | 8.14 | 37.8 | 6 | 6 | - | NC_005322.1 | [102] | ||||||
| pER371 | ST371 | 2.67 | 38.2 | 3 | 3 | - | Rep 247 aa | NC_004968.1 | [103] | ||||
| pER13 | ST113 | RCR | 4.14 | 38.4 | 4 | 4 | - | RepB 217 aa | Mob 499 aa | NC_002776.1 | [104] |
References
- Adolfsson, O.; Meydani, S.N.; Russell, R.M. Yogurt and gut function. Am. J. Clin. Nutr. 2004, 80, 245–256. [Google Scholar] [PubMed]
- Iyer, R.; Tomar, S.K.; Maheswari, T.U.; Singh, R. Streptococcus thermophilus strains: Multifunctional lactic acid bacteria. Int. Dairy J. 2010, 20, 133–141. [Google Scholar] [CrossRef]
- Vaningelgem, F.; Zamfir, M.; Mozzi, F.; Adriany, T.; Vancanneyt, M. Biodiversity of exopolysaccharides produced by Streptococcus thermophilus strains is reflected in their production and their molecular and functional characteristics. Appl. Environ. Microbiol. 2004, 70, 900–912. [Google Scholar] [CrossRef] [PubMed]
- Hols, P.; Hancy, F.; Fontaine, L.; Grossiord, B.; Prozzi, D. New insights in the molecular biology and physiology of Streptococcus thermophilus revealed by comparative genomics. FEMS Microbiol. Rev. 2005, 29, 435–463. [Google Scholar] [PubMed]
- Zotta, T.; Ricciardi, A.; CiociaI, F.; Rossano, R.; Parente, E. Diversity of stress responses in dairy thermophilic streptococci. Int. J. Food Microbiol. 2008, 124, 34–42. [Google Scholar] [CrossRef] [PubMed]
- Galia, W.; Perrin, C.; Genay, M. Variability and molecular typing of Streptococcus thermophilus strains displaying different proteolytic and acidifying properties. Int. Dairy J. 2009, 19, 89–95. [Google Scholar] [CrossRef]
- Vuyst, L.D.; Weckx, S.; Ravyts, F.; Herman, L.; Leroy, F. New insights into the exopolysaccharide production of Streptococcus thermophilus. Int. Dairy J. 2011, 21, 586–591. [Google Scholar] [CrossRef]
- Miclo, L.; Roux, É.; Genay, M.; Brusseaux, É.; Poirson, C.; Jameh, N.; Perrin, C.; Dary, A. Variability of hydrolysis of β-, αs1-, and αs2-Caseins by 10 strains of Streptococcus thermophilus and resulting bioactive peptides. J. Agric. Food Chem. 2012, 60, 554–565. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Marzotto, M.; Cremonese, S.; Rizzotti, L.; Torriani, S. Diversity of Streptococcus thermophilus in bacteriocin production, inhibitory spectrum and occurrence of thermophilin genes. Food Microbiol. 2013, 35, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bolotin, A.; Quinquis, B.; Renault, P.; Sorokin, A.; Ehrlich, S.D.; Kulakauskas, S.; Lapidus, A.; Goltsman, E.; Mazur, M.; Pusch, G.D.; et al. Complete sequence and comparative genome analysis of the dairy bacterium Streptococcus thermophilus. Nat. Biotechnol. 2004, 22, 1554–1558. [Google Scholar] [CrossRef] [PubMed]
- Delorme, C.; Bartholini, C.; Luraschi, M.; Pons, N.; Loux, V.; Almeida, M.; Guédon, E.; Gibrat, J.F.; Renault, P. Complete genome sequence of the pigmented Streptococcus thermophilus strain JIM8232. J. Bacteriol. 2011, 193, 5581–5582. [Google Scholar] [CrossRef] [PubMed]
- Makarova, K.; Slesarev, A.; Wolf, Y.; Sorokin, A.; Mirkin, B.; Koonin, E.; Pavlov, A.; Pavlova, N.; Karamychev, V.; Polouchine, N.; et al. Comparative genomics of the lactic acid bacteria. Proc. Natl. Acad. Sci. USA 2006, 103, 15611–15616. [Google Scholar] [CrossRef] [PubMed]
- Sun, Z.; Chen, X.; Wang, J.; Zhao, W.; Shao, Y.; Wu, L.; Zhou, Z.; Sun, T.; Wang, L.; Meng, H.; et al. Complete genome sequence of Streptococcus thermophilus strain ND03. J. Bacteriol. 2011, 193, 793–794. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Ling, N.; Sun, G.; Zhou, Q.; Zhang, L.; Sheng, Q. Complete genome sequence of Streptococcus thermophilus strain MN-ZLW-002. J. Bacteriol. 2012, 194, 4428–4429. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Tun, H.M.; Leung, F.C.; Shah, N.P. Genomic insights into high exopolysaccharide-producing dairy starter bacterium Streptococcus thermophilus ASCC 1275. Sci. Rep. 2014, 4, 4974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Labrie, S.J.; Tremblay, D.M.; Plante, P.L.; Wasserscheid, J.; Dewar, K.; Corbeil, J.; Moineau, S. Complete genome sequence of Streptococcus thermophilus SMQ-301, a model strain for phage-post interactions. Genome Announc. 2015, 3, e00480-15. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Chen, Y.; Li, Z.; Yang, L.; Chen, W.; Mu, Z. Complete genome sequence of Streptococcus thermophilus MN-BM-A02, a rare strain with a high acid-producing rate and low post-acidification ability. Genome Announc. 2015, 3, e00979-15. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Sun, E.; Shi, Y.D.; Jiang, Y.Y.; Chen, Y.; Liu, S.L.; Zhao, L.; Zhang, M.; Guo, H.Y.; Zhang, H.; et al. Complete genome sequence of Streptococcus thermophilus MN-BM-A01, a strain with high exopolysaccharides production. J. Biotechnol. 2016, 224, 45–46. [Google Scholar] [CrossRef] [PubMed]
- Treu, L.; Vendramin, V.; Bovo, B.; Campanaro, S.; Corich, V.; Giacomini, A. Genome sequences of Streptococcus thermophilus strains MTH17CL396 and M17PTZA496 from Fontina, an Italian PDO cheese. Genome Announc. 2014, 2, e00067-14. [Google Scholar] [CrossRef] [PubMed]
- Treu, L.; Vendramin, V.; Bovo, B.; Campanaro, S.; Corich, V.; Giacominia, A. Whole-genome sequences of Streptococcus thermophilus strains TH1435 and TH1436, isolated from raw goat milk. Genome Announc. 2014, 2, e01129-13. [Google Scholar] [CrossRef] [PubMed]
- Treu, L.; Vendramin, V.; Bovo, B.; Campanaro, S.; Corich, V.; Giacomini, A. Genome sequences of four Italian Streptococcus thermophilus strains of dairy origin. Genome Announc. 2014, 2, e00126-14. [Google Scholar] [CrossRef] [PubMed]
- Prajapati, J.B.; Nathani, N.M.; Patel, A.K.; Senan, S.; Joshi, C.G. Genomic analysis of dairy starter culture Streptococcus thermophilus MTCC 5461. J. Microbiol. Biotechnol. 2013, 23, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Coute-Monvoisin, A.C.; Stahl, B.; Chavichvily, I.; Damange, F.; Romero, D.A.; Boyaval, P.; Fremaux, C.; Horvath, P. Genomic impact of CRISPR immunization against bacteriophages. Biochem. Soc. Trans. 2013, 41, 1383–1391. [Google Scholar] [CrossRef] [PubMed]
- Wels, M.; Serrano, L.M.; Eibrink, B.J.; Backus, L.; Bongers, R.S.; Vriesendorp, B.; Siezen, R.J.; van Hijum, S.A.; Meijer, W.C. Draft genome sequence of Streptococcus thermophilus C106, a dairy isolate from an artisanal cheese produced in the countryside of Ireland. Genome Announc. 2015, 3, e01377-15. [Google Scholar] [CrossRef] [PubMed]
- Goh, Y.J.; Goin, C.; O’Flaherty, S.; Altermann, E.; Hutkins, R. Specialized adaptation of a lactic acid bacterium to the milk environment: The comparative genomics of Streptococcus thermophilus LMD-9. Microb. Cell Factories 2011, 10 (Suppl. 1), 10066–10071. [Google Scholar] [CrossRef] [PubMed]
- Eng, C.; Thibessard, A.; Danielsen, M.; Rasmussen, T.B.; Mari, J.F.; Leblond, P. In silico prediction of horizontal gene transfer in Streptococcus thermophilus. Arch. Microbiol. 2011, 193, 287–297. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.J.; Siezen, R.J.; Nauta, A. In silico prediction of horizontal gene transfer events in Latobacillus bulgaricus and Streptococcus thermophilus reveals protocooperation in yogurt manufacturing. Appl. Environ. Microbiol. 2009, 75, 4120–4129. [Google Scholar] [CrossRef] [PubMed]
- Rasmussen, T.B.; Danielsen, M.; Valina, O.; Garrigues, C.; Johansen, E.; Pedersen, M.B. Streptococcus thermophilus core genome: Comparative genome hybridization study of 47 strains. Appl. Environ. Microbiol. 2008, 74, 4703–4710. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.H.; Hu, T.; Qu, X.J.; Zhang, L.W.; Ding, Z.Q.; Dong, A.J. Plasmids from food lactic acid bacteria: Diversity, similarity, and new developments. Int. J. Mol. Sci. 2015, 16, 13172–13202. [Google Scholar] [CrossRef] [PubMed]
- Mercenier, A. Molecular genetics of Streptococcus thermophilus. FEMS Microbiol. Rev. 1990, 87, 61–78. [Google Scholar] [CrossRef]
- Somkuti, G.A.; Solaiman, D.K.Y.; Steinberg, D.H. Structural and functional properties of the hsp16.4-bearing plasmid pER341 in Streptococcus thermophilus. Plasmid 1998, 40, 61–72. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, T.; van Sinderen, D.; Fitzgerald, G. Structural and functional analysis of pCI65st, a 6.5 kb plasmid from Streptococcus thermophilus NDI-6. Microbiology 1999, 145, 127–134. [Google Scholar] [CrossRef] [PubMed]
- Su, P.; Jury, K.; Allison, G.E.; Wong, W.Y.; Kim, W.S.; Liu, C.Q.; Vancov, T.; Dunn, N.W. Cloning vectors for Streptococcus thermophilus derived from a native plasmid. FEMS Microbiol. Lett. 2002, 216, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Geis, A.E.; Demerdash, H.A.M.; Heller, K.J. Sequence analysis and characterization of plasmids from Streptococcus thermophilus. Plasmid 2003, 50, 53–69. [Google Scholar] [CrossRef]
- Petrova, P.; Miteva, V.; Ruiz-Maso, J.A.; Solar, G.D. Structural and functional analysis of pt38, a 2.9 kb plasmid of Streptococcus thermophilus yoghurt strain. Plasmid 2003, 50, 176–189. [Google Scholar] [CrossRef]
- Somkuti, G.A.; Steinberg, D.H. Promoter activity of the pER341-borne STphsp in heterologous gene expression in E. coli and Streptococcus thermophilus. FEMS Microbiol. Lett. 1999, 179, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Solow, B.T.; Somkuti, G.A. Comparison of low-molecular-weight heat stress proteins encoded on plasmids in different strains of Streptococcus thermophilus. Curr. Microbiol. 2000, 41, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Turgeon, N.; Moineau, S. Isolation and characterization of a Streptococcus thermophilus plasmid closely related to the pMV158 family. Plasmid 2001, 45, 171–183. [Google Scholar] [CrossRef] [PubMed]
- Girard, S.L.; Moineau, S. Analysis of two θ-replicating plasmids of Streptococcus thermophilus. Plasmid 2007, 58, 174–181. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, V.S.; Woychik, J.H. Utilization of lactose, glucose, and galactose by a mixed culture of Streptococcus thermophilus and Lactobacillus bulgaricus in milk treated with lactase enzyme. Appl. Environ. Microbiol. 1976, 32, 89–94. [Google Scholar] [PubMed]
- Tinson, W.; Ratcliff, M.F.; Hillier, A.J.; Jago, G.R. Metabolism of Streptococcus thermophilus. 3. Influence on the level of bacterial metabolites in Cheddar cheese. Aust. J. Dairy Technol. 1982, 37, 17–21. [Google Scholar]
- De Vin, F.; Rådström, P.; Herman, L.; de Vuyst, L. Molecular and biochemical analysis of the galactose phenotype of dairy Streptococcus thermophilus strains reveals four different fermentation profiles. J. Appl. Microbiol. 2005, 71, 3659–3667. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, K.K. Isolation of galactose-fermenting thermophilic cultures and their use in the manufacture of low browning Mozzarella cheese. J. Dairy Sci. 1994, 77, 2839–2849. [Google Scholar] [CrossRef]
- Giraffa, G.; Paris, A.; Valcavi, L.; Gatti, M.; Neviani, E. Genotypic and phenotypic heterogeneity of Streptococcus thermophilus strains isolated from dairy products. J. Appl. Microbiol. 2001, 91, 937–943. [Google Scholar] [CrossRef] [PubMed]
- Mora, D.; Fortina, M.G.; Parini, C.; Ricci, G.; Gatti, M.; Giraffa, G.; Manachini, P.L. Genetic diversity and technological properties of Streptococcus thermophilus strains isolated from dairy products. J. Appl. Microbiol. 2002, 93, 278–287. [Google Scholar] [CrossRef] [PubMed]
- Morandi, S.; Brasca, M. Safety aspects, genetic diversity and technological characterisation of wild-type Streptococcus thermophilus strains isolated from north Italian traditional cheeses. Food Control 2012, 23, 203–209. [Google Scholar] [CrossRef]
- Erkus, O.; Okuklu, B.; Yenidunya, A.F.; Harsa, S. High genetic and phenotypic variability of Streptococcus thermophilus strains isolated from artisanal Yuruk yoghurts. LWT-Food Sci. Technol. 2014, 58, 348–354. [Google Scholar] [CrossRef]
- Thomas, M.; Wrzosek, L.; Ben-Yahia, L.; Noordine, M.-L.; Gitton, C.; Chevret, D.; Langella, P.; Mayeur, C.; Cherbuy, C.; Rul, F. Carbohydrate metabolism is essential for the colonization of Streptococcus thermophilus in the digestive tract of gnotobiotic rats. PLoS ONE 2011, 6, e28789. [Google Scholar] [CrossRef] [PubMed]
- Maxwell, E.S.; Kurahasi, K.; Kalckar, H.M. Enzymes of the Leloir pathway. Methods Enzymol. 1962, 5, 174–189. [Google Scholar]
- Vaughan, E.E.; van den Bogaard, P.T.; Catzeddu, P.; Kuipers, O.P.; de Vos, W.M. Actiation of silent gal genes in the lac-gal regulon of Streptococcus thermophilus. J. Bacteriol. 2001, 183, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Vaillancourt, K.; LeMay, J.D.; Lamoureux, M.; Frenette, M.; Moineau, S.; Vadeboncoeur, C. Characterization of a galactokinase-positive recombinant strain of Streptococcus thermophilus. Appl. Environ. Microbiol. 2004, 70, 4596–4603. [Google Scholar] [CrossRef] [PubMed]
- Van den Bogaard, R.T.C.; Hols, P.; Kuipers, O.P.; Kleerebezem, M.; de Vos, W.M. Sugar utilisation and conservation of the gal-lac gene cluster in Streptococcus thermophilus. Syst. Appl. Microbiol. 2004, 27, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Anbukkarasi, K.; Nanda, D.K.; UmaMaheswari, T.; Hemalatha, T.; Singh, P.; Singh, R. Assessment of expression of Leloir pathway genes in wild-type galactose-fermenting Streptococcus thermophilus by real-time PCR. Eur. Food Res. Technol. 2014, 239, 895–903. [Google Scholar] [CrossRef]
- Sørensen, K.I.; Curic-Bawden, M.; Junge, M.P.; Janzen, T.; Johansen, E. Enhancing the sweetness of yoghurt through metabolic remodeling of carbohydrate metabolism in Streptococcus thermophilus and Lactobacillus delbrueckii subsp. bulgaricus. Appl. Environ. Microbiol. 2016, 82, 3683–3692. [Google Scholar] [CrossRef] [PubMed]
- Levander, F.; Rådström, P. Requirement for phosphoglucomutase in exopolysaccharide biosynthesis in glucose- and lactose-utilizing Streptococcus thermophilus. Appl. Environ. Microbiol. 2001, 67, 2734–2738. [Google Scholar] [CrossRef] [PubMed]
- Levander, F.; Svensson, M.; Rådström, P. Enhanced exopolysaccharide production by metabolic engineering of Streptococcus thermophilus. Appl. Environ. Microbiol. 2002, 68, 784–790. [Google Scholar] [CrossRef] [PubMed]
- Hirasuka, Y.; Li, G. Alcohol and eye diseases: A review of epidemiologic studies. J. Stud. Alcohol. 1992, 62, 372–402. [Google Scholar]
- Broadbent, J.R.; Mcmahon, D.J.; Welker, D.L.; Oberg, C.J.; Moineau, S. Biochemistry, genetics, and applications of exopolysaccharide production in Streptococcus thermophilus: A review. J. Dairy Sci. 2003, 86, 407–423. [Google Scholar] [CrossRef]
- Hassan, A.N.; Frank, J.F.; Schmidt, K.A.; Shalab, S.I. Rheological properties of yogurt made with encapsulated non ropy lactic cultures. J. Dairy Sci. 1996, 79, 2091–2097. [Google Scholar] [CrossRef]
- Bhaskaracharya, R.K.; Shah, N.P. Texture characteristics and microstructure of skim milk mozzarella cheeses made using exopolysaccharide or non-exopolysaccharide producing starter cultures. Aust. J. Dairy Technol. 2000, 55, 132–138. [Google Scholar]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Syneresis in set yogurt as affected by EPS starter cultures and levels of solids. Int. J. Dairy Technol. 2006, 59, 216–221. [Google Scholar] [CrossRef]
- Purwandari, U.; Shah, N.P.; Vasiljevic, T. Effects of exopolysaccharide producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. Int. Dairy J. 2007, 17, 1344–1352. [Google Scholar] [CrossRef]
- Marcial, G.; Messing, J.; Menchicchi, B.; Goycoolea, F.M.; Faller, G.; de Valdez Graciela, F.; Hensel, A. Effects of polysaccharide isolated from Streptococcus thermophilus CRL1190 on human gastric epithelial cells. Int. J. Biol. Macromol. 2013, 62, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Ren, W.; Xia, Y.; Wang, G.; Zhang, H.; Zhu, S.; Ai, L. Bioactive exopolysaccharides from a S. thermophilus strain: Screening, purification and characterization. Int. J. Biol. Macromol. 2016, 86, 402–407. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Medici, M.; Mozzi, F.; de Valdez Graciela, F. Therapeutic effect of Streptococcus thermophilus CRL 1190-fermented milk on chronic gastritis. World J. Gastroenterol. 2010, 16, 1622–1630. [Google Scholar] [CrossRef] [PubMed]
- Ruas-Madiedo, P.; Abraham, A.; Mozzi, F.; Los Reyes-Gavilán, C.G.D. Functionality of exopolysaccharides produced by lactic acid bacteria. In Molecular Aspects of Lactic Acid Bacteria for Traditional and New Applications; Mayo, B., Lόpez, P., Pérez-Martínez, G., Eds.; Research Signpost: Kerala, India, 2008; pp. 137–166. [Google Scholar]
- Laws, A.; Gu, Y.C.; Marshall, V. Biosynthesis, characterisation and design of bacterial exopolysaccharides from lactic acid bacteria. Biotechnol. Adv. 2001, 19, 597–625. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, Y.; Wang, J.; Guo, X.; Zheng, Y.; Zhao, W.; Mei, X.; Guo, T.; Yang, Z. Physicochemical characteristics and bioactivities of the exopolysaccharide and its sulphated polymer from Streptococcus thermophilus GST-6. Carbohydr. Polym. 2016, 146, 368–375. [Google Scholar] [CrossRef] [PubMed]
- Mozzi, F.; Vaningelgem, F.; Hebert, E.M.; Meulen, R.V.D.; Moreno, M.R.F.; Valdez, G.F.D.; Vuyst, L.D. Diversity of heteropolysaccharide-producing lactic acid bacterium strains and their biopolymers. Appl. Environ. Microbiol. 2006, 72, 4431–4435. [Google Scholar] [CrossRef] [PubMed]
- Jolly, L.; Stingele, F. Molecular organization and functionality of exopolysaccharide gene clusters in lactic acid bacteria. Int. Dairy J. 2001, 11, 733–745. [Google Scholar] [CrossRef]
- Stingele, F.; Neeser, J.R.; Mollet, B. Identification and characterization of the eps (exopolysaccharide) gene cluster from Streptococcus thermophilus Sfi6. J. Bacteriol. 1996, 178, 1680–1690. [Google Scholar] [PubMed]
- Kranenburg, R.V.; Marugg, J.D.; Swam, I.I.V.; Willem, N.J.; Devos, W.M. Molecular characterization of the plasmid encoded eps gene cluster essential for exopolysaccharide biosynthesis in Lactococcus lactis. Mol. Microbiol. 1997, 24, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Bourgoin, F.; Pluvinet, A.; Gintz, B.; Decaris, B.; Guédon, G. Are horizontal transfers involved in the evolution of the Streptococcus thermophilus exopolysaccharide synthesis loci? Gene 1999, 233, 151–161. [Google Scholar] [CrossRef]
- Stingele, F.; Vincent, S.J.F.; Faber, E.J.; Newell, J.W.; Kamerling, J.P.; Neeser, J.R. Introduction of the exopolysaccharide gene cluster from Streptococcus thermophilus, Sfi6 into Lactococcus lactis, MG1363: Production and characterization of an altered polysaccharide. Mol. Microbiol. 1999, 32, 1287–1295. [Google Scholar] [CrossRef] [PubMed]
- Van Kranenburg, R.; Vos, H.R.; Swam, I.I.V.; Kleerebezem, M.; de Vos, W.M. Functional analysis of glycosyltransferase genes from Lactococcus lactis and other gram-positive cocci: Complementation, expression, and diversity. J. Bacteriol. 1999, 181, 6347–6353. [Google Scholar] [PubMed]
- Ruas-Madiedo, P.; Los Reyes-Gavilán, C.G.D. Invited Review: Methods for the screening, isolation, and characterization of exopolysaccharides produced by lactic acid bacteria. J. Dairy Sci. 2005, 88, 843–856. [Google Scholar] [CrossRef]
- Zisu, B.; Shah, N.P. Effects of pH, temperature, supplementation with whey protein concentrate, and adjunct cultures on the production of exopolysaccharides by Streptococcus thermophilus 1275. J. Dairy Sci. 2003, 86, 3405–3415. [Google Scholar] [CrossRef]
- Amatayakul, T.; Sherkat, F.; Shah, N.P. Physical characteristics of set yoghurt made with altered casein to whey protein ratios and EPS-producing starter cultures at 9 and 14% total solids. Food Hydrocoll. 2006, 20, 314–324. [Google Scholar] [CrossRef]
- Zisu, B.; Shah, N.P. Low-fat mozzarella as influenced by microbial exopolysaccharides, preacidification, and whey protein concentrate. J. Dairy Sci. 2005, 88, 1973–1985. [Google Scholar] [CrossRef]
- Degeest, B.; Vaningelgem, F.; Vuyst, L.D. Microbial physiology fermentation kinetics, and process engineering of heteropolysaccharide production by lactic acid bacteria. Int. Dairy J. 2001, 11, 747–757. [Google Scholar] [CrossRef]
- Li, D.; Li, J.; Zhao, F.; Wang, G.H.; Qin, Q.Q.; Hao, Y.L. The influence of fermentation condition on production and molecular mass of EPS produced by Streptococcus thermophilus 05–34 in milk-based medium. Food Chem. 2016, 197, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Pébay, M.; Colmin, C.; Guédon, G.; Simonet, J.M.; Decaris, B. Chromosomal genetic instability in S. thermophilus. Lait Dairy Sci. Technol. 1993, 73, 181–190. [Google Scholar] [CrossRef]
- Savijoki, K.; Ingmer, H.; Varmanen, P. Proteolytic systems of lactic acid bacteria. Appl. Microbiol. Biotechnol. 2006, 71, 394–406. [Google Scholar] [CrossRef] [PubMed]
- Christensen, J.E.; Dudley, E.G.; Pederson, J.A.; Steele, J.L. Peptidases and amino acid catabolism in lactic acid bacteria. Antonie Van Leeuwenhoek 1999, 76, 217–246. [Google Scholar] [CrossRef] [PubMed]
- Shihata, A.; Shah, N.P. Proteolytic profiles of yogurt and probiotic bacteria. Int. Dairy J. 2000, 10, 401–408. [Google Scholar] [CrossRef]
- Liu, M.J.; Bayjanov, J.R.; Renckens, B.; Nauta, A.; Siezen, R.J. The proteolytic system of lactic acid bacteria revisited: A genomic comparison. BMC Genom. 2010, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Settachaimongkon, S.; Nout, M.J.R.; Fernandes, E.C.A.; Hettinga, K.A.; Vervoort, J.M.; van Hooijdonk, T.C.M.; Zwietering, M.H.; Smid, E.J.; van Valenberg, H.J.F. Influence of different proteolytic strains of Streptococcus thermophilus in co-culture with Lactobacillus delbrueckii subsp. bulgaricus on the metabolite profile of set-yoghurt. Int. J. Food Microbiol. 2014, 177, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chang, O.K.; Perrin, C.; Galia, W.; Saulnier, F.; Miclo, L.; Roux, E.; Driou, A.; Humbert, G.; Dary, A. Release of the cell-envelope protease PrtS in the growth medium of Streptococcus thermophilus 4F44. Int. Dairy J. 2012, 23, 91–98. [Google Scholar] [CrossRef]
- Shahbal, S.; Hemme, D.; Desmazeaud, M. High cell wall-associated proteinase activity of some Streptococcus thermophilus strains (H-strains) correlated with a high acidification rate in milk. Le Lait 1991, 71, 351–357. [Google Scholar] [CrossRef]
- Mora, D.; Ricci, G.; Guglielmetti, S.; Daffonchio, D.; Fortina, M.G. 16S–23S rRNA intergenic spacer region sequence variation in Streptococcus thermophilus and related dairy streptococci and development of a multiplex ITS-SSCP analysis for their identification. Microbiology 2003, 149, 807–813. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H. Volatile flavor compounds in yogurt: A review. Crit. Rev. Food Sci. Nutr. 2010, 50, 938–950. [Google Scholar] [CrossRef] [PubMed]
- Ott, A.; Fay, L.B.; Chaintreau, A. Determination and origin of the aroma impact compounds of yogurt flavor. J. Agric. Food Chem. 1997, 45, 850–858. [Google Scholar] [CrossRef]
- Imhof, R.; Bosset, J.O. Relationships between microorganisms and formation of aroma compounds in fermented dairy products. Z. Lebensm. Unters. Forsch. 1994, 198, 267–276. [Google Scholar] [CrossRef]
- Liu, M.J.; Nauta, A.; Francke, C.; Siezen, R.J. Comparative genomics of enzymes in flavor-forming pathways from amino acids in lactic acid bacteria. Appl. Environ. Micobol. 2008, 74, 4590–4600. [Google Scholar] [CrossRef] [PubMed]
- Yvon, M.; Rijnen, L. Cheese flavour formation by amino acid catabolism. Int. Dairy J. 2001, 11, 185–201. [Google Scholar] [CrossRef]
- Smit, G.; Smit, B.A.; Engels, W.J.M. Flavour formation by lactic acid bacteria and biochemical flavour profiling of cheese products. FEMS Microbiol. Rev. 2005, 29, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Ardo, Y. Flavour formation by amino acid catabolism. Biotechnol. Adv. 2006, 24, 238–242. [Google Scholar] [CrossRef] [PubMed]
- Helinck, S.; Le Bars, D.; Moreau, D.; Yvon, M. Ability of thermophilic lactic acid bacteria to produce aroma compounds from amino acids. Appl. Environ. Micobol. 2004, 70, 3855–3861. [Google Scholar] [CrossRef] [PubMed]
- Akyol, I.; Ozcelik, F.G.; Karakas-Sen, A.; Ozkose, E.; Gezginc, Y.; Ekinci, M.S. Cloning and over expression of the als, pflA, and adhB genes in Streptococcus thermophilus and their effects on metabolite formation. Mol. Biotechnol. 2015, 57, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.J. Characteristics of Acid and Flavor-Producing Stretococcus thermophilus and Lactobacillus bulgaricus, as well as Their Functional Gene Typing and Expression. Ph.D. Thesis, Inner Mongolia Agricultural University, Hohhot, China, 2014. [Google Scholar]
- Liu, W.J.; Yu, J.; Sun, Z.H.; Song, Y.Q.; Wang, X.N.; Wang, H.M.; Wuren, T.Y.; Zha, M.S.; Menghe, B.; Zhang, H.P. Relationships between functional genes in Lactobacillus delbrueckii ssp. bulgaricus isolates and phenotypic characteristics associated with fermentation time and flavor production in yogurt elucidated using multilocus sequence typing. J. Dairy Sci. 2016, 99, 89–103. [Google Scholar] [PubMed]
- Turgeon, N.; Frenette, M.; Moineau, S. Characterization of a θ-replicating plasmid from Streptococcus thermophilus. Plasmid 2004, 51, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Solaiman, D.K.; Somkuti, G.A. Characterization of a novel Streptococcus thermophilus rolling-circle plasmid used for vector construction. Appl. Microbiol. Biotechnol. 1998, 50, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Somkuti, G.A.; Steinberg, D.H. Molecular organization of plasmid pER13 in Streptococcus thermophilus. Biotechnol. Lett. 2007, 29, 1991–1999. [Google Scholar] [CrossRef] [PubMed]


© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, Y.; Xu, T.; Qu, X.; Hu, T.; Jiang, X.; Zhao, C. New Insights into Various Production Characteristics of Streptococcus thermophilus Strains. Int. J. Mol. Sci. 2016, 17, 1701. https://doi.org/10.3390/ijms17101701
Cui Y, Xu T, Qu X, Hu T, Jiang X, Zhao C. New Insights into Various Production Characteristics of Streptococcus thermophilus Strains. International Journal of Molecular Sciences. 2016; 17(10):1701. https://doi.org/10.3390/ijms17101701
Chicago/Turabian StyleCui, Yanhua, Tingting Xu, Xiaojun Qu, Tong Hu, Xu Jiang, and Chunyu Zhao. 2016. "New Insights into Various Production Characteristics of Streptococcus thermophilus Strains" International Journal of Molecular Sciences 17, no. 10: 1701. https://doi.org/10.3390/ijms17101701