Neurotoxicity of a Biopesticide Analog on Zebrafish Larvae at Nanomolar Concentrations
Abstract
:1. Introduction
2. Results
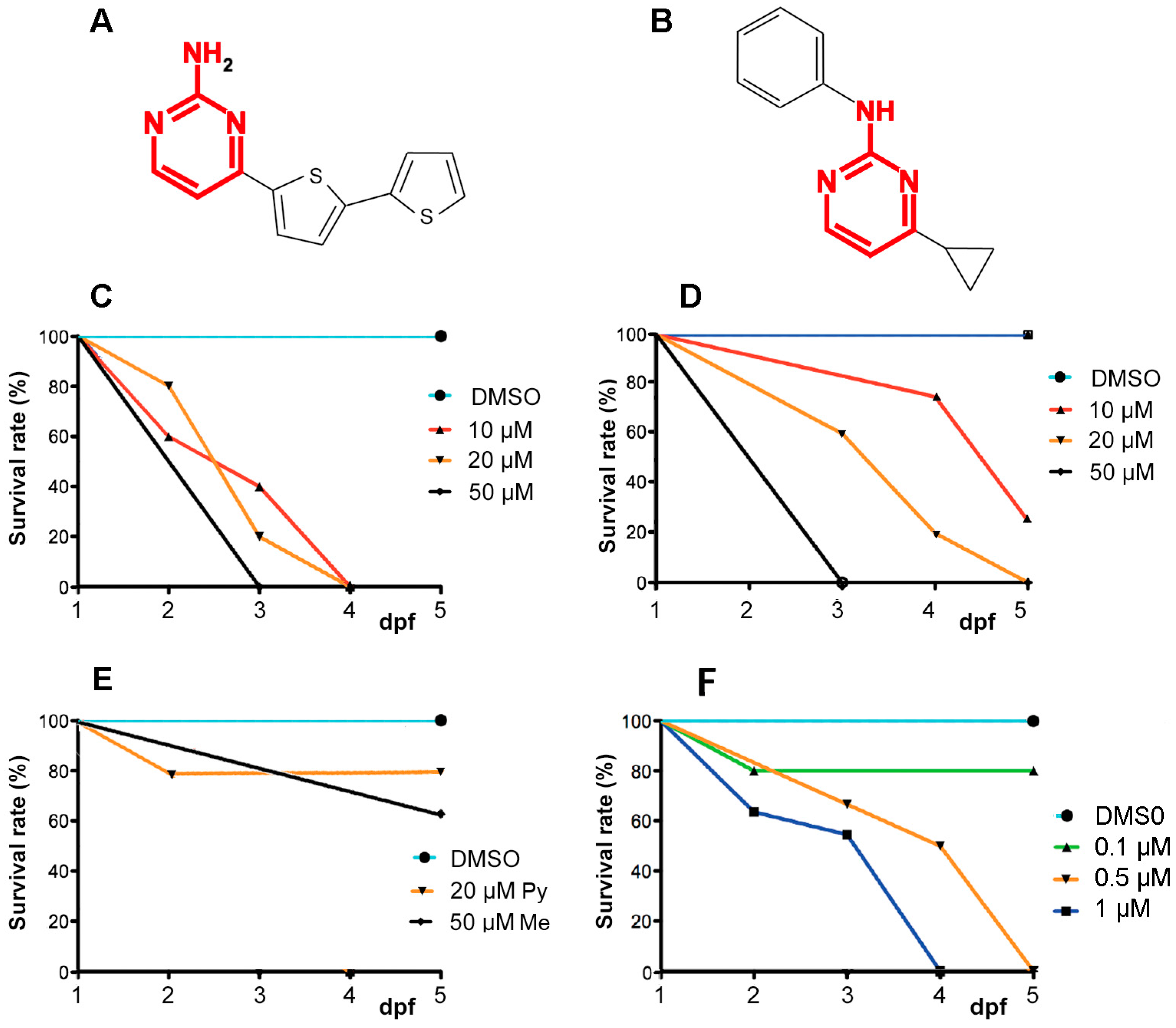
2.1. A6 and Cyprodinyl Induce Lethality and Behavioral Defects in Zebrafish Larvae
2.2. Effect of A6 and Cyprodinyl on Spinal Cord Neurons
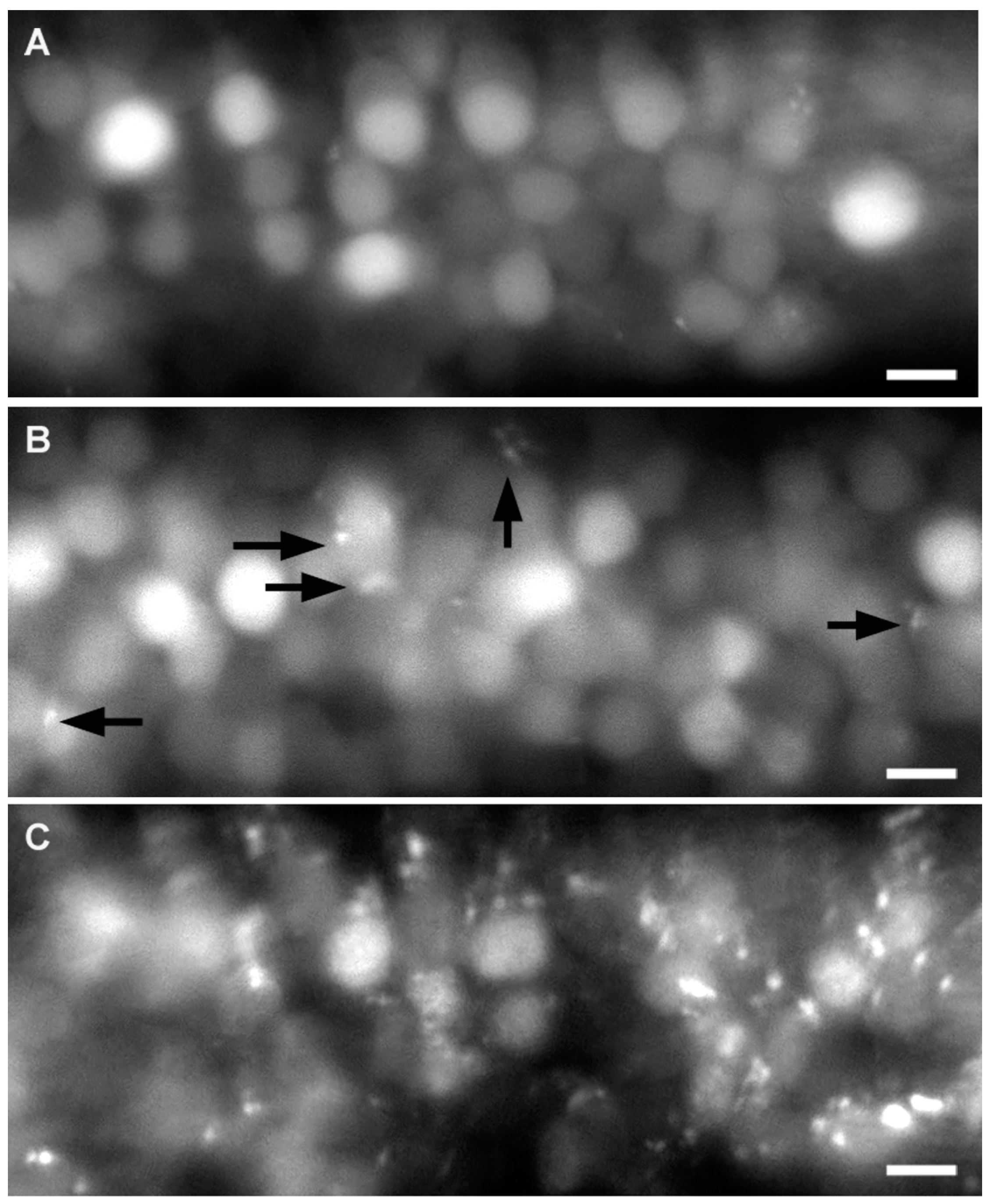
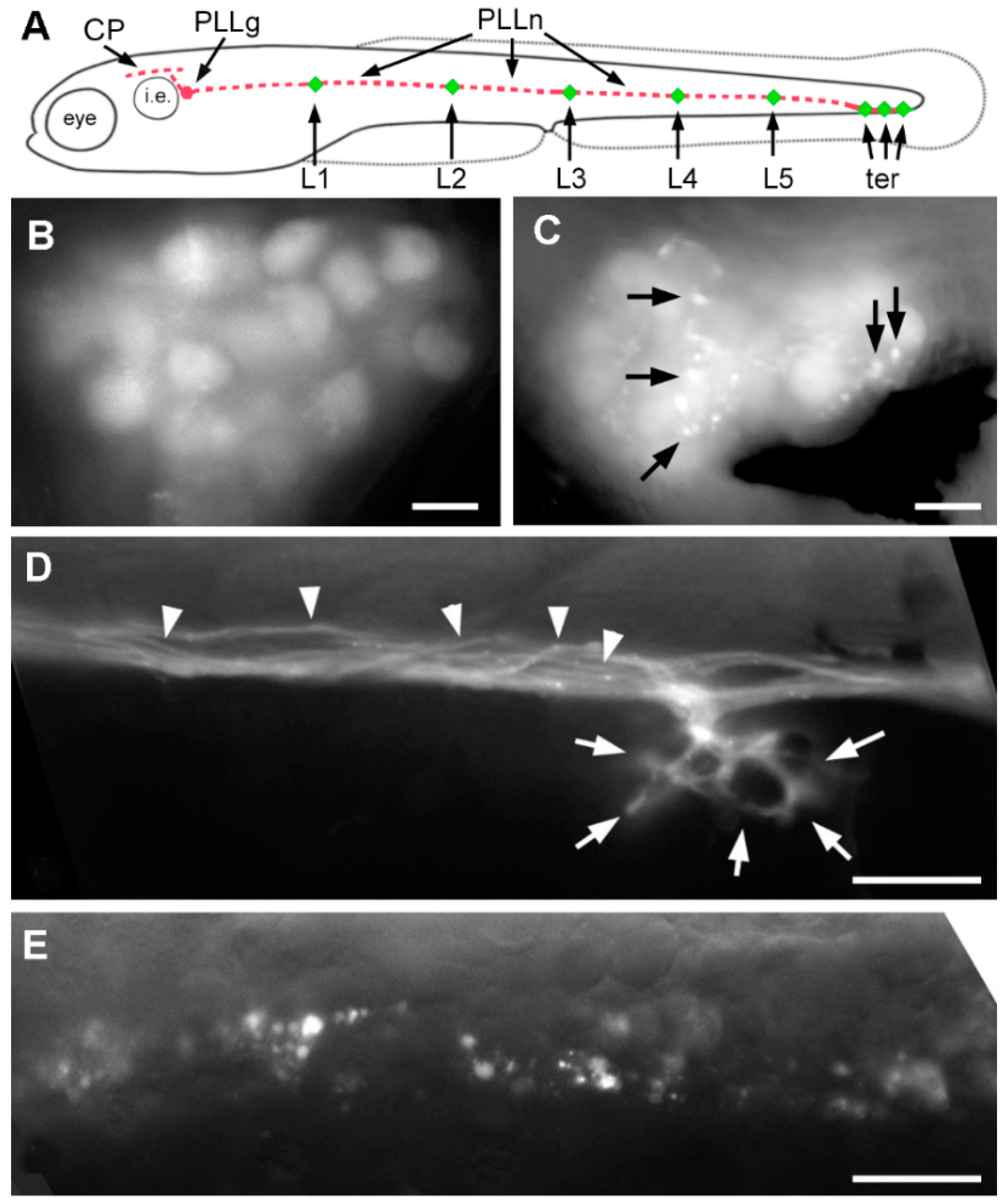
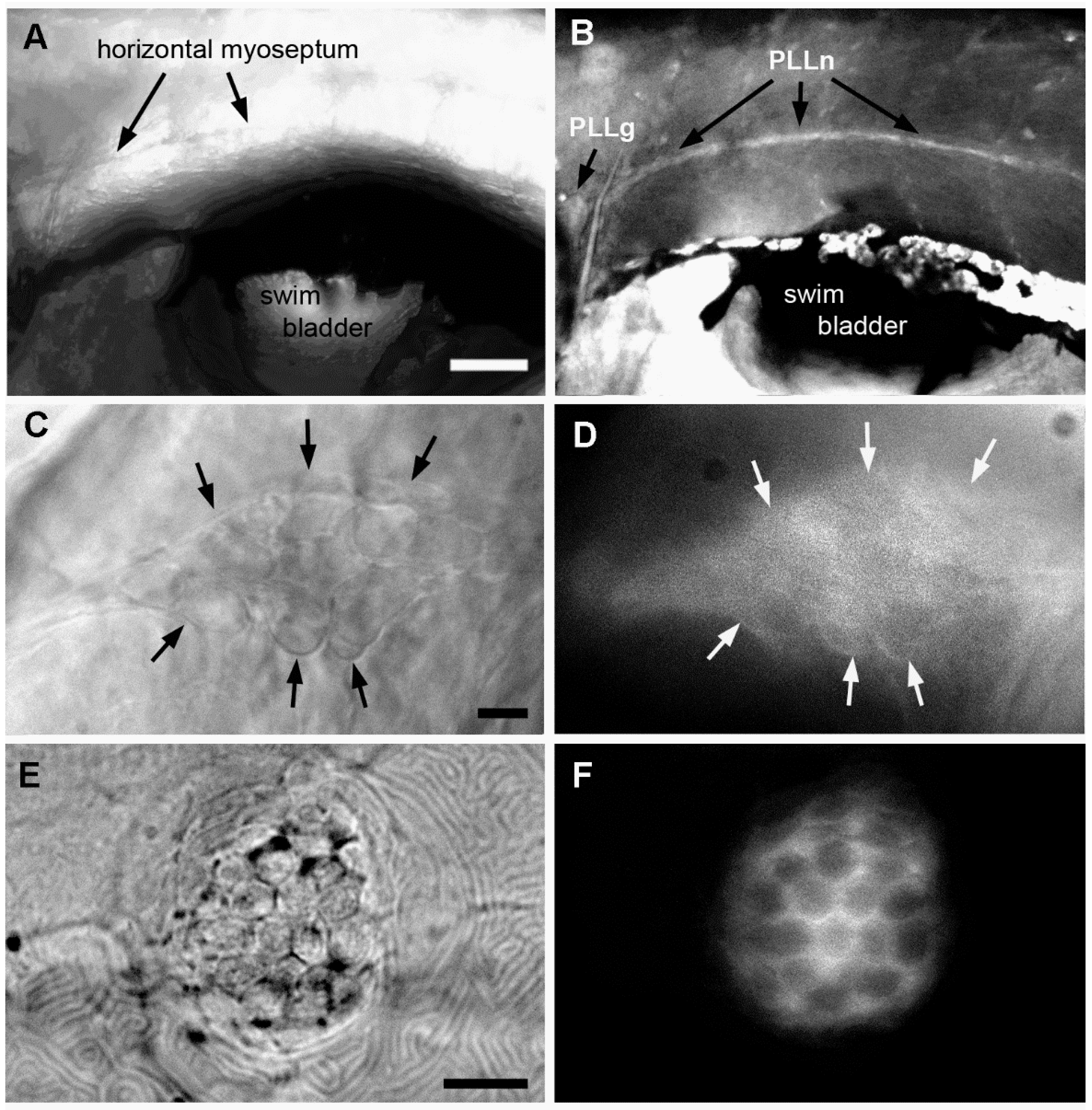
2.3. Effect of A6 on the Lateral Line System
2.4. Neural Accumulation of A6 in the Lateral Line System
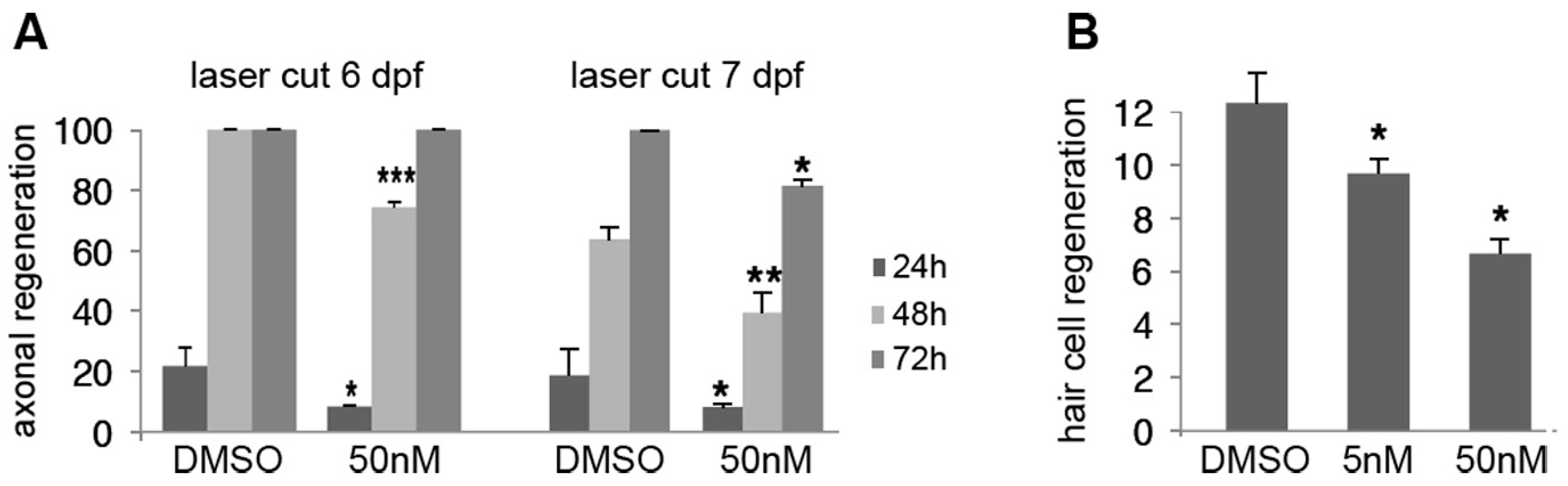
2.5. Effect of Low Doses of A6 on Axon and Hair Cell Regeneration
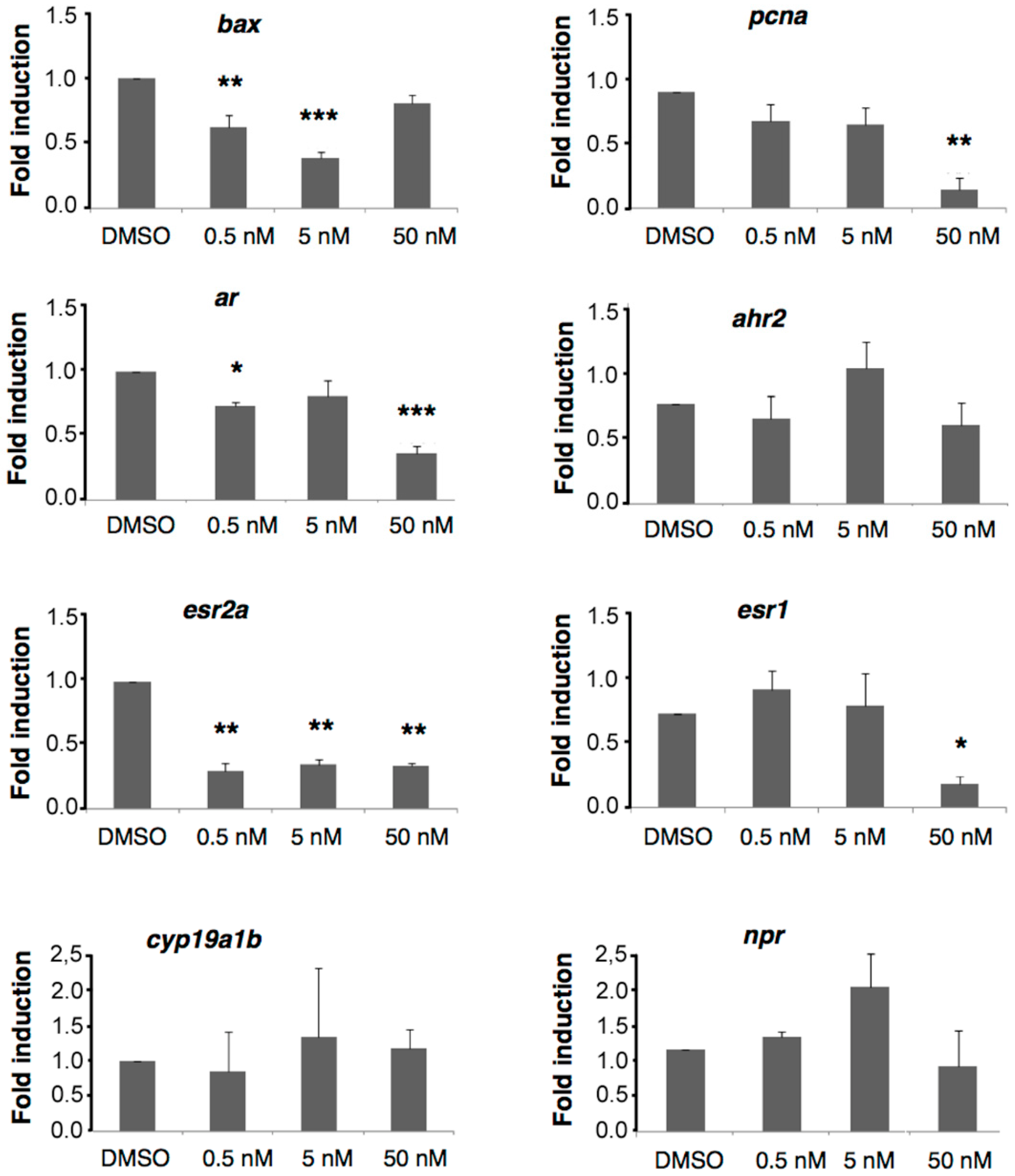
2.6. A6 Down-Regulates Gene Expression
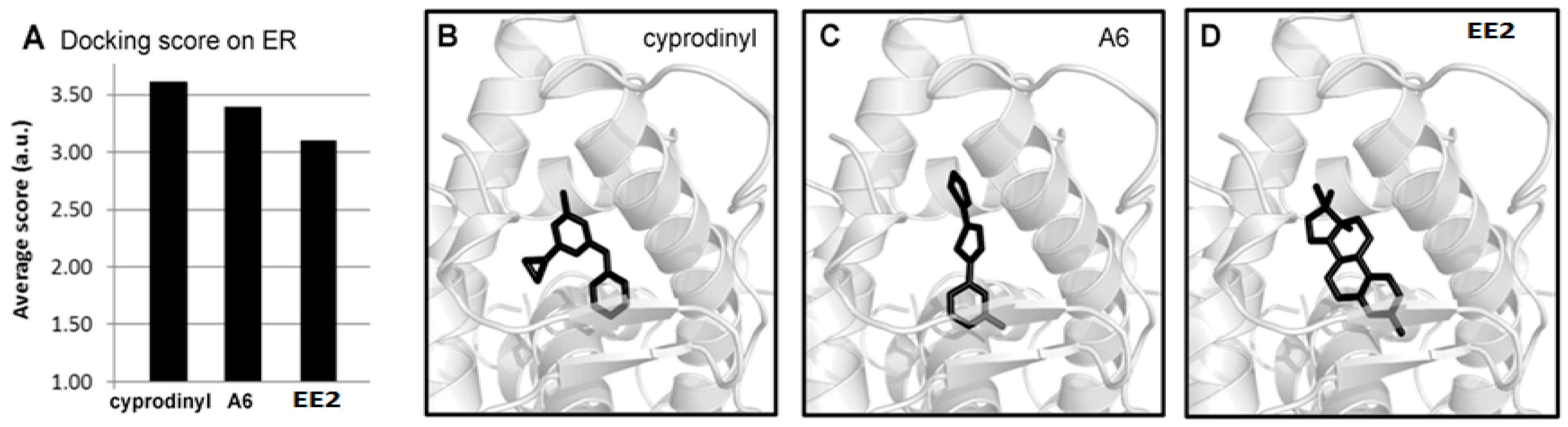
2.7. Molecular Modeling Studies of A6 and Cyprodinyl Binding on Estrogen Receptors
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Fish Strains and Treatment of Embryos
4.3. Pesticides
4.4. Survival Curves and Behavioral Observations
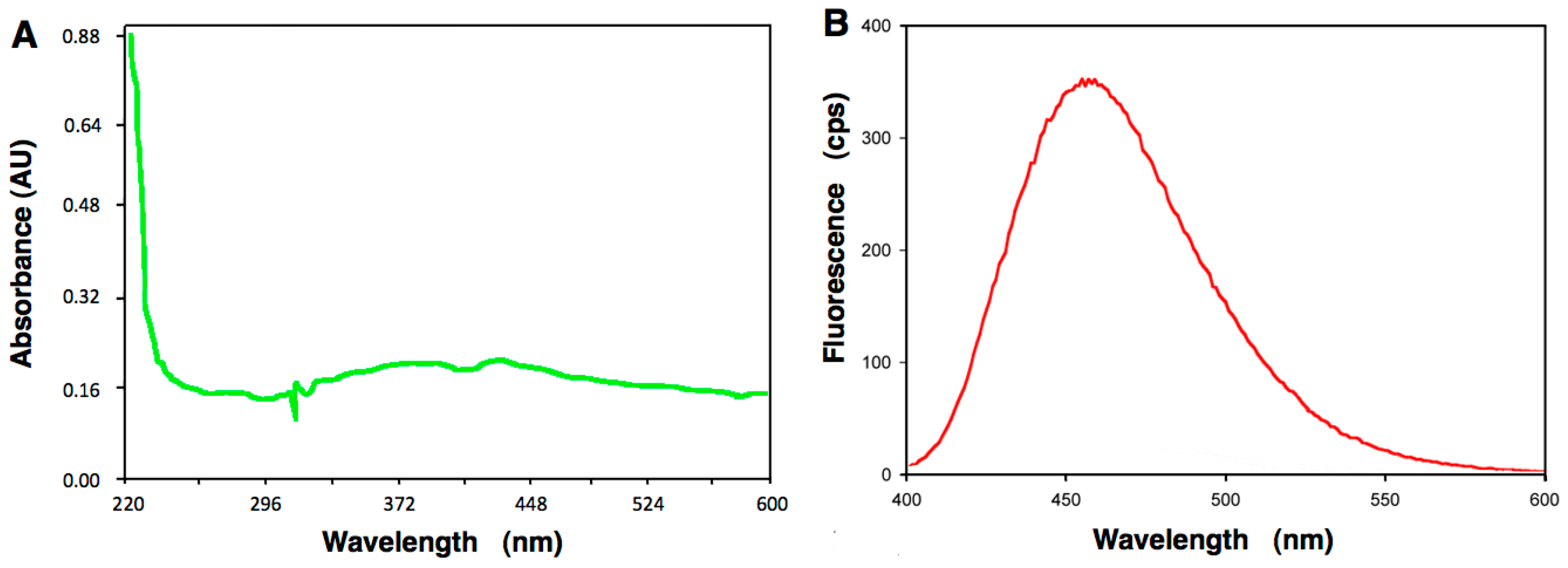
4.5. Absorption Spectroscopy of A6 and Fluorescence Studies
4.6. Neural Accumulation
4.7. Axonal Regeneration
4.8. Hair Cell Regeneration
4.9. Determination of Gene Expression Levels by Quantitative Real-Time PCR
4.9.1. Embryo Exposure
4.9.2. RNA Isolation
4.9.3. qRT-PCR Analyses: esr1, esr2a, ar, npr, ahr2, cyp19a1b, pcna, bax and ef1a
4.10. Data Analysis
4.11. Molecular Modeling Studies
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Schwarzenbach, R.P.; Escher, B.I.; Fenner, K.; Hofstetter, T.B.; Johnson, C.A.; von Gunten, U.; Wehrli, B. The challenge of micropollutants in aquatic systems. Science 2006, 313, 1072–1077. [Google Scholar] [CrossRef] [PubMed]
- United Nations Educational, Scientific, and Cultural Organization; World Water Assesment Programme. Water for People, Water for Life—The United Nations World Water Development Report; Bergham Books: Barcelona, Spain, 2003. [Google Scholar]
- Huang, G.Y.; Liu, Y.S.; Chen, X.W.; Liang, Y.Q.; Liu, S.S.; Yang, Y.Y.; Hu, L.X.; Shi, W.J.; Tian, F.; Zhao, J.L.; et al. Feminization and masculinization of western mosquitofish (Gambusia affinis) observed in rivers impacted by municipal wastewaters. Sci. Rep. 2016, 6, 20884. [Google Scholar] [CrossRef] [PubMed]
- Ibor, O.R.; Adeogun, A.O.; Fagbohun, O.A.; Arukwe, A. Gonado-histopathological changes, intersex and endocrine disruptor responses in relation to contaminant burden in Tilapia species from Ogun River, Nigeria. Chemosphere 2016, 164, 248–262. [Google Scholar] [CrossRef] [PubMed]
- Harris, C.A.; Hamilton, P.B.; Runnalls, T.J.; Vinciotti, V.; Henshaw, A.; Hodgson, D.; Coe, T.S.; Jobling, S.; Tyler, C.R.; Sumpter, J.P. The consequences of feminization in breeding groups of wild fish. Environ. Health Perspect. 2011, 119, 306–311. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Sampaio, D.R.; Paris, F.; Audran, F.; Orsini, M.; Neto, J.B.; Sultan, C. High prevalence of micropenis in 2710 male newborns from an intensive-use pesticide area of Northeastern Brazil. Int. J. Androl. 2012, 35, 253–264. [Google Scholar] [CrossRef] [PubMed]
- Gaspari, L.; Paris, F.; Jeandel, C.; Sultan, C. Peripheral precocious puberty in a 4-month-old girl: Role of pesticides? Gynecol. Endocrinol. 2011, 27, 721–724. [Google Scholar] [CrossRef] [PubMed]
- Paris, F.; Gaspari, L.; Servant, N.; Philibert, P.; Sultan, C. Increased serum estrogenic bioactivity in girls with premature thelarche: A marker of environmental pollutant exposure? Gynecol. Endocrinol. 2013, 29, 788–792. [Google Scholar] [CrossRef] [PubMed]
- Luc, D.S.; Benoit, A.; Laurette, D.; Michel, M. Indirect evidence that agricultural pesticides select for insecticide resistance in the malaria vector Anopheles gambiae. J. Vector Ecol. 2016, 41, 34–40. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Li, K.; Wang, X.; Yang, X.; Lin, Y.; Cai, F.; Zhong, W.; Lin, C.; Lin, Z.; Ma, Y. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou City, Hainan Island, China. Infect. Dis. Poverty 2016, 5, 31. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Canale, A.; Toniolo, C.; Higuchi, A.; Murugan, K.; Pavela, R.; Nicoletti, M. Neem (Azadirachta indica): Towards the ideal insecticide? Nat. Prod. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Campos, E.V.R.; de Oliveira, J.L.; Pascoli, M.; de Lima, R.; Fraceto, L.F. Neem oil and crop protection: From now to the future. Front. Plant Sci. 2016, 7, 1494. [Google Scholar] [CrossRef] [PubMed]
- Murugan, K.; Panneerselvam, C.; Subramaniam, J.; Madhiyazhagan, P.; Hwang, J.S.; Wang, L.; Dinesh, D.; Suresh, U.; Roni, M.; Higuchi, A.; et al. Eco-friendly drugs from the marine environment: Spongeweed-synthesized silver nanoparticles are highly effective on Plasmodium falciparum and its vector Anopheles stephensi, with little non-target effects on predatory copepods. Environ. Sci. Pollut. Res. Int. 2016, 23, 16671–16685. [Google Scholar] [CrossRef] [PubMed]
- Zechmeister, L.; Sease, J.W. A blue-fluorescing compound, terthienyl, isolated from marigolds. J. Am. Chem. Soc. 1947, 69, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Friedman, D.C.S.; Friedman, P. A theoretical study of 2,2′,5′,2′′-terthiophene (α-T) and its analogs. Part 1. Correlation of electronic structure and energies with herbicidal phototoxicity. J. Mol. Struct. 1995, 333, 71–78. [Google Scholar] [CrossRef]
- Nagata, T.; Masuda, K.; Maeno, S.; Miura, I. Synthesis and structure-activity study of fungicidal anilinopyrimidines leading to mepanipyrim (KIF-3535) as an anti-Botrytis agent. Pest Manag. Sci. 2004, 60, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Ayrolles-Torro, A.; Imberdis, T.; Torrent, J.; Toupet, K.; Baskakov, I.V.; Poncet-Montange, G.; Grégoire, C.; Roquet-Baneres, F.; Lehmann, S.; Rognan, D.; et al. Oligomeric-induced activity by thienyl pyrimidine compounds traps prion infectivity. J. Neurosci. 2011, 31, 14882–14892. [Google Scholar] [CrossRef] [PubMed]
- Imberdis, T.; Ayrolles-Torro, A.; Verdier, J.-M.; Perrier, V. Thienyl pyrimidine derivatives with PrPSc oligomer-inducing activity are a promising tool to study prions. Curr. Top. Med. Chem. 2013, 13, 2477–2483. [Google Scholar] [CrossRef] [PubMed]
- Imberdis, T.; Ayrolles-Torro, A.; Duarte Rodrigues, A.; Torrent, J.; Alvarez-Martinez, M.T.; Kovacs, G.G.; Verdier, J.-M.; Robitzer, M.; Perrier, V. A Fluorescent Oligothiophene-Bis-Triazine ligand interacts with PrP fibrils and detects SDS-resistant oligomers in human prion diseases. Mol. Neurodegener. 2016, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Medjakovic, S.; Zoechling, A.; Gerster, P.; Ivanova, M.M.; Teng, Y.; Klinge, C.M.; Schilberger, B.; Gartner, M.; Jungbauer, A. Effect of nonpersistent pesticides on estrogen receptor, androgen receptor, and aryl hydrocarbon receptor. Environ. Toxicol. 2014, 29, 1201–1216. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.-H.; Wang, Y.-H.; Wu, Y.-H. Developmental exposures to ethanol or dimethylsulfoxide at low concentrations alter locomotor activity in larval zebrafish: Implications for behavioral toxicity bioassays. Aquat. Toxicol. 2011, 102, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Ghysen, A.; Dambly-Chaudière, C. Development of the zebrafish lateral line. Curr. Opin. Neurobiol. 2004, 14, 67–73. [Google Scholar] [CrossRef] [PubMed]
- Ghysen, A.; Dambly-Chaudière, C. The lateral line microcosmos. Genes Dev. 2007, 21, 2118–2130. [Google Scholar] [CrossRef] [PubMed]
- Graciarena, M.; Dambly-Chaudière, C.; Ghysen, A. Dynamics of axonal regeneration in adult and aging zebrafish reveal the promoting effect of a first lesion. Proc. Natl. Acad. Sci. USA 2014, 111, 1610–1615. [Google Scholar] [CrossRef] [PubMed]
- Owens, K.N.; Coffin, A.B.; Hong, L.S.; Bennett, K.O.; Rubel, E.W.; Raible, D.W. Response of mechanosensory hair cells of the zebrafish lateral line to aminoglycosides reveals distinct cell death pathways. Hear. Res. 2009, 253, 32–41. [Google Scholar] [CrossRef] [PubMed]
- Olivari, F.A.; Hernández, P.P.; Allende, M.L. Acute copper exposure induces oxidative stress and cell death in lateral line hair cells of zebrafish larvae. Brain Res. 2008, 1244, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Kniss, J.S.; Jiang, L.; Piotrowski, T. Insights into sensory hair cell regeneration from the zebrafish lateral line. Curr. Opin. Genet. Dev. 2016, 40, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Williams, J.A.; Holder, N. Cell turnover in neuromasts of zebrafish larvae. Hear. Res. 2000, 143, 171–181. [Google Scholar] [CrossRef]
- Korsmeyer, S.J.; Gross, A.; Harada, H.; Zha, J.; Wang, K.; Yin, X.M.; Wei, M.; Zinkel, S. Death and survival signals determine active/inactive conformations of pro-apoptotic BAX, BAD, and BID molecules. Cold Spring Harb. Symp. Quant. Biol. 1999, 64, 343–350. [Google Scholar] [CrossRef] [PubMed]
- Malikov, V.; Madeira, M.D. Regulation of ERα protein expression by 17β-estradiol in cultured neurons of hypothalamic ventromedial nucleus. Neurochem. Res. 2013, 38, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Mouriec, K.; Gueguen, M.-M.; Manuel, C.; Percevault, F.; Thieulant, M.-L.; Pakdel, F.; Kah, O. Androgens upregulate cyp19a1b (aromatase B) gene expression in the brain of zebrafish (Danio rerio) through estrogen receptors. Biol. Reprod. 2009, 80, 889–896. [Google Scholar] [CrossRef] [PubMed]
- Diotel, N.; Servili, A.; Gueguen, M.-M.; Mironov, S.; Pellegrini, E.; Vaillant, C.; Zhu, Y.; Kah, O.; Anglade, I. Nuclear progesterone receptors are up-regulated by estrogens in neurons and radial glial progenitors in the brain of zebrafish. PLoS ONE 2011, 6, e28375. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delfosse, V.; Grimaldi, M.; Cavaillès, V.; Balaguer, P.; Bourguet, W. Structural and functional profiling of environmental ligands for estrogen receptors. Environ. Health Perspect. 2014, 122, 1306–1313. [Google Scholar] [CrossRef] [PubMed]
- Rand, G. Fundamentals of Aquatic Toxicology: Effects, Environment Fate and Risk Assesments, 2nd ed.; Taylor & Francis: Washington, DC, USA, 1995. [Google Scholar]
- Crosby, E.B.; Bailey, J.M.; Oliveri, A.N.; Levin, E.D. Neurobehavioral impairments caused by developmental imidacloprid exposure in zebrafish. Neurotoxicol. Teratol. 2015, 49, 81–90. [Google Scholar] [CrossRef] [PubMed]
- Melo, K.M.; Oliveira, R.; Grisolia, C.K.; Domingues, I.; Pieczarka, J.C.; de Souza Filho, J.; Nagamachi, C.Y. Short-term exposure to low doses of rotenone induces developmental, biochemical, behavioral, and histological changes in fish. Environ. Sci. Pollut. Res. Int. 2015, 22, 13926–13938. [Google Scholar] [CrossRef] [PubMed]
- Kung, T.S.; Richardson, J.R.; Cooper, K.R.; White, L.A. Developmental deltamethrin exposure causes persistent changes in dopaminergic gene expression, neurochemistry, and locomotor activity in zebrafish. Toxicol. Sci. 2015, 146, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Bakker, J.; Gommers, F.J.; Nieuwenhuis, I.; Wynberg, H. Photoactivation of the nematicidal compound α-terthienyl from roots of marigolds (Tagetes species). A possible singlet oxygen role. J. Biol. Chem. 1979, 254, 1841–1844. [Google Scholar] [PubMed]
- Revankar, C.M.; Cimino, D.F.; Sklar, L.A.; Arterburn, J.B.; Prossnitz, E.R. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science 2005, 307, 1625–1630. [Google Scholar] [CrossRef] [PubMed]
- Filardo, E.; Quinn, J.; Pang, Y.; Graeber, C.; Shaw, S.; Dong, J.; Thomas, P. Activation of the novel estrogen receptor G protein-coupled receptor 30 (GPR30) at the plasma membrane. Endocrinology 2007, 148, 3236–3245. [Google Scholar] [CrossRef] [PubMed]
- Maggiolini, M.; Picard, D. The unfolding stories of GPR30, a new membrane-bound estrogen receptor. J. Endocrinol. 2010, 204, 105–114. [Google Scholar] [CrossRef] [PubMed]
- Brailoiu, E.; Dun, S.L.; Brailoiu, G.C.; Mizuo, K.; Sklar, L.A.; Oprea, T.I.; Prossnitz, E.R.; Dun, N.J. Distribution and characterization of estrogen receptor G protein-coupled receptor 30 in the rat central nervous system. J. Endocrinol. 2007, 193, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Liu, X.; Zhu, P.; Li, J.; Sham, K.W.Y.; Cheng, S.H.; Li, S.; Zhang, Y.; Cheng, C.H.K.; Lin, H. G-protein-coupled estrogen receptor 1 is involved in brain development during zebrafish (Danio rerio) embryogenesis. Biochem. Biophys. Res. Commun. 2013, 435, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Kodavanti, P.R.S. Neurotoxicity of persistent organic pollutants: Possible mode(s) of action and further considerations. Dose Response 2006, 3, 273–305. [Google Scholar] [CrossRef] [PubMed]
- Schreiber, T.; Gassmann, K.; Götz, C.; Hübenthal, U.; Moors, M.; Krause, G.; Merk, H.F.; Nguyen, N.H.; Scanlan, T.S.; Abel, J.; et al. Polybrominated diphenyl ethers induce developmental neurotoxicity in a human in vitro model: Evidence for endocrine disruption. Environ. Health Perspect. 2010, 118, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Westerfield, M. The Zebrafish Book. A Guide for the Laboratory Use of Zebrafish (Danio rerio), 4th ed.; University of Oregon Press: Eugene, Oregon, 2000. [Google Scholar]
- Peri, F.; Nüsslein-Volhard, C. Live imaging of neuronal degradation by microglia reveals a role for v0-ATPase a1 in phagosomal fusion in vivo. Cell 2008, 133, 916–927. [Google Scholar] [CrossRef] [PubMed]
- DeCarvalho, A.C.; Cappendijk, S.L.T.; Fadool, J.M. Developmental expression of the POU domain transcription factor Brn-3b (Pou4f2) in the lateral line and visual system of zebrafish. Dev. Dyn. 2004, 229, 869–876. [Google Scholar] [CrossRef] [PubMed]
- Ingebretson, J.J.; Masino, M.A. Quantification of locomotor activity in larval zebrafish: Considerations for the design of high-throughput behavioral studies. Front. Neural Circuits 2013, 7, 109. [Google Scholar] [CrossRef] [PubMed]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef] [PubMed]
- McCurley, A.T.; Callard, G.V. Characterization of housekeeping genes in zebrafish: Male-female differences and effects of tissue type, developmental stage and chemical treatment. BMC Mol. Biol. 2008, 9, 102. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Dodd, A.; Lai, D.; McNabb, W.C.; Love, D.R. Validation of zebrafish (Danio rerio) reference genes for quantitative real-time RT-PCR normalization. Acta Biochim. Biophys. Sin. 2007, 39, 384–390. [Google Scholar] [CrossRef] [PubMed]
- Jones, G.; Willett, P.; Glen, R.C. Molecular recognition of receptor sites using a genetic algorithm with a description of desolvation. J. Mol. Biol. 1995, 245, 43–53. [Google Scholar] [CrossRef]
- Korb, O.; Stützle, T.; Exner, T.E. Empirical scoring functions for advanced protein-ligand docking with PLANTS. J. Chem. Inf. Model 2009, 49, 84–96. [Google Scholar] [CrossRef] [PubMed]
| Gene Name | Genebank Number | Reverse (rev) and Forward (fw) Primers |
|---|---|---|
| ahr2 | Genbank accession number 105762.2 | (fw) 5′-GAAGAAGCCCGTTCAGAAAA-3′ (rev) 5′-GGGTTGGATTTCACACCATC-3′ |
| ar | Genbank accession number 1083123.1 | (fw) 5′-CACTACGGAGCCCTCACTTGCGGA-3′ (rev) 5′-GCCCTGAACTGCTCCGACCTC-3′ |
| bax | Genbank accession number BC55592 | (fw) 5′-GAGCTGCACTTCTCAACAACTTT-3′ (rev) 5′-CTGGTTGAAATAGCCTTGATGAC-3′ |
| cyp19a1b | Genbank accession number 131642.1 | (fw) 5′-TCGGCACGGCGTGCAACTAC-3′ (rev) 5′-CATACCTATGCATTGCAGACC-3′ |
| ef1a | Genbank accession number 131263.1 | (fw) 5′-AGCAGCAGCTGAGGAGTGAT-3′ (rev) 5′-CCGCATTTGTAGATCAGATGG-3′ |
| esr1 | Genbank accession number 152959.1 | (fw) 5′-CTGGAGATGCTGGACGCTCA-3′ (rev) 5′-GCTGCAGCTCCTCCTCCTGG-3′ |
| esr2a | Genbank accession number 180966.2 | (fw) 5′-GATCCTGCTCAACTCTAATAAC-3′ (rev) 5′-CCAGCAGATTCAGCACCTTCCC-3′ |
| pcna | Genbank accession number BC49535.1 | (fw) 5′-CTCACAGACCAGCAACGTCG-3′ (rev) 5′-GGACAGAGGAGTGGCTTTGG-3′ |
| pr | Genbank accession number EF155644.1 | (fw) 5′-GAGCAATGATCAGCTGAGAAGG-3’ (rev) 5′-TCCAGAGGAACAGTGTTGAGG-3′ |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasri, A.; Valverde, A.J.; Roche, D.B.; Desrumaux, C.; Clair, P.; Beyrem, H.; Chaloin, L.; Ghysen, A.; Perrier, V. Neurotoxicity of a Biopesticide Analog on Zebrafish Larvae at Nanomolar Concentrations. Int. J. Mol. Sci. 2016, 17, 2137. https://doi.org/10.3390/ijms17122137
Nasri A, Valverde AJ, Roche DB, Desrumaux C, Clair P, Beyrem H, Chaloin L, Ghysen A, Perrier V. Neurotoxicity of a Biopesticide Analog on Zebrafish Larvae at Nanomolar Concentrations. International Journal of Molecular Sciences. 2016; 17(12):2137. https://doi.org/10.3390/ijms17122137
Chicago/Turabian StyleNasri, Ahmed, Audrey J. Valverde, Daniel B. Roche, Catherine Desrumaux, Philippe Clair, Hamouda Beyrem, Laurent Chaloin, Alain Ghysen, and Véronique Perrier. 2016. "Neurotoxicity of a Biopesticide Analog on Zebrafish Larvae at Nanomolar Concentrations" International Journal of Molecular Sciences 17, no. 12: 2137. https://doi.org/10.3390/ijms17122137






