A High Redox Potential Laccase from Pycnoporus sanguineus RP15: Potential Application for Dye Decolorization
Abstract
:1. Introduction
2. Results and Discussion
2.1. Preliminary Screening for the Best Carbon Source, Inducer and Supplementary Nitrogen and Carbon Sources for the Production of Laccases by P. sanguineus RP15
2.2. Optimization of Laccase Production by P. sanguineus RP 15 Using RSM
2.3. Lacps1 Purification and Molecular Properties
2.4. Effects of pH and Temperature on Laccase Activity
2.5. Thermal and pH Stabilities of Lacps1
2.6. Kinetic Properties and the Effects of Metals and Inhibitors on the Laccase Activity
2.7. UV–Vis Absorption Spectrum of Lacps1
2.8. Determination of the Redox Potential of the T1 Copper Site of Lacps1
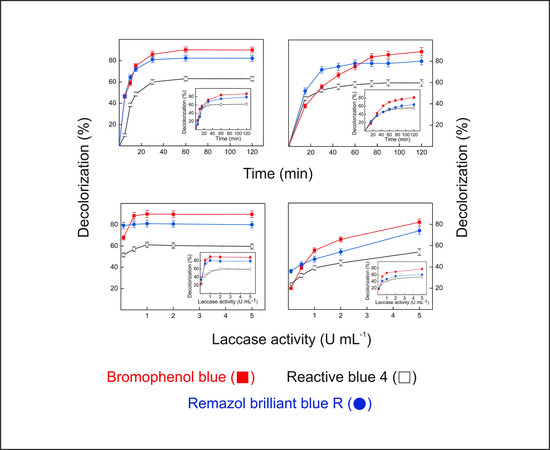
2.9. Dye Decolorization by Lacps1
3. Materials and Methods
3.1. Organism and Strain Maintenance
3.2. Preliminary Screening for the Best Carbon Source, Inducer and Supplementary Nitrogen and Carbon Sources for the Production of Laccases by P. sanguineus RP15 under SSF Conditions
3.3. Response Surface Methodology for the Optimization of the Production of Laccases
3.4. Enzyme Extraction
3.5. Enzymatic Assays
3.6. Purification of Lacps1
3.7. Estimation of Protein and Neutral Carbohydrates
3.8. Polyacrylamide Gel Electrophoresis
3.9. Estimation of the Apparent Molecular Mass of the Native Laccase
3.10. Characterization of the Purified Lacps1 by Mass Spectrometry Analysis
3.11. Effects of Temperature and pH on the Enzymatic Activity of the Purified Lacps1
3.12. Thermal and pH Stabilities of the Lacps1
3.13. Experimental Design, Statistical Analysis and RSM Modeling
3.14. Determination of the Kinetic Parameters and Data Fitting
3.15. UV–Vis Absorption Spectrum of Lacps1
3.16. Determination of the Redox Potential of Lacps1
3.17. Decolorization of Synthetic Dyes by the Laccase-Rich Crude Extract and Pure Lacps1
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Arora, D.S.; Sharma, R.K. Ligninolytic fungal laccases and their biotechnological applications. Appl. Biochem. Biotechnol. 2010, 160, 1760–1788. [Google Scholar] [CrossRef] [PubMed]
- Giardina, P.; Faraco, V.; Pezzella, C.; Piscitelli, A.; Vanhulle, S.; Sannia, G. Laccases: A never-ending story. Cell. Mol. Life Sci. 2010, 67, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Janusz, G.; Kucharzyk, K.H.; Pawlik, A.; Staszczak, M.; Paszczynski, A.J. Fungal laccase, manganese peroxidase and lignin peroxidase: Gene expression and regulation. Enzym. Microb. Technol. 2013, 52, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Rivera-Hoyos, C.M.; Morales-Álvarez, E.D.; Potou-Piñales, R.A.; Pedroza-Rodríguez, A.M.; Rodríguez-Vázquez, R.; Delgado-Boada, J.M. Fungal Laccases. Fungal Biol. Rev. 2013, 27, 67–82. [Google Scholar] [CrossRef]
- Yadav, M.; Yadav, H.S. Applications of ligninolytic enzymes to pollutants, wastewater, dyes, soil, coal, paper and polymers. Environ. Chem. Lett. 2015, 13, 309–318. [Google Scholar] [CrossRef]
- Pezzella, C.; Guarino, L.; Piscitelli, A. How to enjoy laccases. Cell. Mol. Life Sci. 2015, 72, 923–940. [Google Scholar] [CrossRef] [PubMed]
- Uzan, E.; Nousiainen, P.; Balland, V.; Sipila, J.; Piumi, F.; Navarro, D.; Asther, M.; Record, E.; Lomascolo, A. High redox potential laccases from the lignolytic fungi Pycnoporus coccineus and Pycnoporus sanguineus suitable for white biotechnology: From gene cloning to enzyme characterization and applications. J. Appl. Microbiol. 2010, 108, 2199–2213. [Google Scholar] [PubMed]
- Yang, J.; Yang, X.; Lin, Y.; Ng, T.B.; Lin, J.; Ye, X. Laccase-Catalyzed Decolorization of Malachite Green: Performance Optimization and Degradation Mechanism. PLoS ONE 2015, 10, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Sun, H.; Zhang, S.; Wu, B.; Pan, B. Potential of acetylacetone as a mediator for Trametes versicolor laccase in enzymatic transformation of organic pollutants. Environ. Sci. Pollut. Res. 2015, 22, 10882–10889. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Bhawana, P.; Fulekar, M.H. Microbial decolorization and degradation of synthetic dyes: A review. Rev. Environ. Sci. Biotechnol. 2013, 12, 75–97. [Google Scholar] [CrossRef]
- Rai, H.S.; Bhattacharyya, M.S.; Singh, J.; Bansal, T.K.; Vats, P.; Banerjee, U.C. Removal of Dyes from the Effluent of Textile and Dyestuff Manufacturing Industry: A Review of Emerging Techniques With Reference to Biological Treatment. Crit. Rev. Environ. Sci. Technol. 2015, 35, 219–238. [Google Scholar] [CrossRef]
- Epolito, W.J.; Lee, Y.H.; Bottomely, L.A.; Pavlostathis, S.G. Characterization of the textile anthraquinone dye Reactive Blue 4. Dyes Pigment. 2005, 67, 35–46. [Google Scholar] [CrossRef]
- Hsu, C.A.; Wen, T.N.; Su, Y.C.; Jiang, Z.B.; Chen, C.W.; Shyur, L.F. Biological Degradation of Anthroquinone and Azo Dyes by a Novel Laccase from Lentinus sp. Environ. Sci. Technol. 2012, 46, 5109–5117. [Google Scholar] [CrossRef] [PubMed]
- Yao, J.; Jia, R.; Zheng, L.; Wang, B. Rapid Decolorization of Azo Dyes by Crude Manganese Peroxidase from Schizophyllum sp. F17 in Solid-state Fermentation. Biotechnol. Bioprocess Eng. 2013, 18, 868–877. [Google Scholar] [CrossRef]
- Gassara, F.; Brar, S.K.; Tyagi, R.D.; Rojan, P.J.; Verma, M.; Valero, J.R. Parameter optimization for production of ligninolytic enzymes using agro-industrial wastes by response surface method. Biotechnol. Bioprocess Eng. 2011, 16, 343–351. [Google Scholar] [CrossRef]
- Xin, F.; Geng, A. Utilization of horticultural waste for laccase production by Trametes versicolor under solid-state fermentation. Appl. Biochem. Biotechnol. 2011, 163, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.; Zhao, M.; Zhang, B.B.; Yu, S.Y.; Bian, X.J.; Wang, W.; Yan, W. Purification and characterization of laccase from Pycnoporus sanguineus and decolorization of an anthraquinone dye by the enzyme. Appl. Microbiol. Biotechnol. 2007, 74, 1232–1239. [Google Scholar] [CrossRef] [PubMed]
- Lomascolo, A.; Uzan-Boukhris, E.; Herpoël-Gimbert, I.; Sigoillot, J.C.; Lesage-Meessen, L. Peculiarities of Pycnoporus species for applications in biotechnology. Appl. Microbiol. Biotechnol. 2011, 92, 1129–1149. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Ornelas-Soto, N.; Cárdenas-Chávez, D.L.; Hernández-Luna, C.; Demarche, P.; Enaud, E.; García-Morales, R.; Agathos, N.; Parra, R. Purification and characterization of two thermostable laccases from Pycnoporus sanguineus and potential role in degradation of endocrine disrupting chemicals. J. Mol. Catal. B-Enzym. 2014, 108, 32–42. [Google Scholar] [CrossRef]
- Wang, Z.X.; Cai, Y.J.; Liao, X.R.; Tao, G.J.; Li, Y.Y.; Zhang, F.; Zhang, D.B. Purification and characterization of two thermostable laccases with high cold adapted characteristics from Pycnoporus sp. SYBC-L1. Process Biochem. 2010, 45, 1720–1729. [Google Scholar] [CrossRef]
- Gutiérrez-Soto, G.; Medina-González, G.E.; García-Zambrano, E.A.; Trevinõ-Ramírez, J.E.; Hernández-Luna, C.E. Selection and Characterization of a Native Pycnoporus sanguineus Strain as a Lignocellulolytic Extract Producer from Submerged Cultures of Various Agroindustrial Wastes. BioResources 2015, 10, 3564–3576. [Google Scholar] [CrossRef]
- Ramírez-Cavazos, L.I.; Junghanns, C.; Nair, R.; Cárdenas-Chávez, D.L.; Hernández-Luna, C.; Agathos, S.N.; Parra, R. Enhanced production of thermostable laccases from a native strain of Pycnoporus sanguineus using central composite design. Biomed. Biotechnol. 2014, 15, 343–352. [Google Scholar]
- Eugenio, M.E.; Carbajo, J.M.; Martín, J.A.; González, A.E.; Villar, J.C. Laccase production by Pycnoporus sanguineus under different culture conditions. J. Basic Microb. 2009, 49, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Singhania, R.R.; Patel, A.K.; Soccol, C.R.; Pandey, A. Recent advances in solid-state fermentation. Biochem. Eng. J. 2009, 44, 13–18. [Google Scholar] [CrossRef]
- Barrios-González, J. Solid-state fermentation: Physiology of solid medium, its molecular basis and applications. Process Biochem. 2012, 47, 175–185. [Google Scholar] [CrossRef]
- Thomas, L.; Larroche, C.; Pandey, A. Current developments in solid-state fermentation. Biochem. Eng. J. 2013, 81, 146–161. [Google Scholar] [CrossRef]
- Zimbardi, A.L.R.L.; Sehn, C.; Meleiro, L.P.; Souza, F.H.M.; Masui, D.C.; Nozawa, M.S.F.; Guimarães, L.H.S.; Jorge, J.A.; Furriel, R.P.M. Optimization of β-Glucosidase, β-Xylosidase and Xylanase Production by Colletotrichum graminicola under Solid-State Fermentation and Application in Raw Sugarcane Trash Saccharification. Int. J. Mol. Sci. 2013, 14, 2875–2902. [Google Scholar] [CrossRef] [PubMed]
- Vikineswary, S.; Abdullah, N.; Renuvathani, M.; Sekaran, M.; Pandey, A.; Jones, E.B.G. Productivity of laccase in solid substrate fermentation of selected agro-residues by Pycnoporus sanguineus. Bioresour. Technol. 2006, 97, 171–177. [Google Scholar] [CrossRef] [PubMed]
- Munusamy, U.; Vikineswary, S.; Muniandy, S.; Abdullah, N.; Pandey, A.; Jones, E.B.G. Biodegradation of Polycyclic Aromatic Hydrocarbons by Laccase of Pycnoporus sanguineus and Toxicity Evaluation of Treated PAH. Biotechnology 2008, 7, 669–677. [Google Scholar]
- Annuar, M.S.M.; Murthy, S.S.; Vikineswary, S. Laccase production from oil palm industry solid waste: Statistical optimization of selected process parameters. Eng. Life Sci. 2010, 10, 40–48. [Google Scholar] [CrossRef]
- Gioia, L.; Manta, C.; Ovsejevi, K.; Burgueño, J.; Menéndez, P.; Rodriguez-Couto, S. Enhancing laccase production by a newly-isolated strain of Pycnoporus sanguineus with high potential for dye decolouration. RSC Adv. 2014, 4, 34096–34103. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Exploitation of biological wastes for the production of value-added products under solid-state fermentation conditions. Biotechnol. J. 2008, 3, 859–870. [Google Scholar] [CrossRef] [PubMed]
- Zilly, A.; Bazanella, G.C.S.; Helm, C.V.; Araújo, C.A.V.; Souza, C.G.M.; Bracht, A.; Peralta, R.M. Solid-State Bioconversion of Passion Fruit Waste by White-Rot Fungi for Production of Oxidative and Hydrolytic Enzymes. Food Bioprocess Technol. 2012, 5, 1573–1580. [Google Scholar] [CrossRef]
- Bazanella, G.C.S.; Souza, D.F.; Castoldi, R.; Oliveira, R.F.; Bracht, A.; Peralta, R.M. Production of laccase and manganese peroxidase by Pleurotus pulmonarius in solid-state culture and application in dye decolorization. Folia Microbiol. 2013, 58, 641–647. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.M.N.; Kyazze, G.; Keshavarz, T. Advances in the Valorization of Lignocellulosic Materials by Biotechnology: An Overview. BioResources 2013, 8, 3157–3176. [Google Scholar] [CrossRef]
- Majeau, J.A.; Brar, S.K.; Tyagi, R.D. Laccases for removal of recalcitrant and emerging pollutants. Bioresour. Technol. 2010, 101, 2331–2350. [Google Scholar] [CrossRef] [PubMed]
- Papinutti, V.L.; Diorio, L.A.; Forchassin, F. Production of laccase and manganese peroxidase by Fomes sclerodermeus grown on wheat bran. J. Ind. Microbiol. Biotechnol. 2003, 30, 157–160. [Google Scholar] [CrossRef] [PubMed]
- Revankar, M.S.; Desais, K.M.; Lele, S.S. Solid-state Fermentation for Enhanced Production of Laccase using Indigenously Isolated Ganoderma sp. Appl. Biochem. Biotechnol. 2007, 143, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Neifar, M.; Kamoun, A.; Jaouani, A.; Ghorbel, R.E.; Chaabouni, S.E. Application of Asymetrical and Hoke Designs for Optimization of Laccase Production by the White-Rot Fungus Fomes fomentarius in Solid-State Fermentation. Enzym. Res. 2011, 2011, 1–12. [Google Scholar]
- Machuca, A.; Aoyama, H.; Duran, N. Production and characterization of thermostable phenol oxidases of the ascomycete Thermoascus aurantiacus. Biotechnol. Appl. Biochem. 1998, 27, 217–223. [Google Scholar]
- Neifar, M.; Jaouani, A.; Ellouze-Ghorbel, R.; Ellouze-Chaabouni, S.; Penninckx, M.J. Effect of culturing processes and copper addition on laccase production by the white-rot fungus Fomes fomentarius MUCL 35117. Lett. Appl. Microbiol. 2009, 49, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Piscitelli, A.; Giardina, P.; Lettera, V.; Pezzella, C.; Sannia, G.; Faraco, V. Induction and Transcriptional Regulation of Laccases in Fungi. Curr. Genom. 2011, 12, 104–112. [Google Scholar] [CrossRef] [PubMed]
- Meza, J.C.; Auria, R.; Lomascolo, A.; Sigoillot, J.C.; Casalot, L. Role of ethanol on growth, laccase production and protease activity in Pycnoporus cinnabarinus ss3. Enzym. Microb. Technol. 2007, 41, 162–168. [Google Scholar] [CrossRef]
- Pant, D.; Adholeya, A. Identification, Ligninolytic Enzyme Activity and Decolorization Potential of Two Fungi Isolated from a Distillery Effluent Contaminated Site. Water Air Soil Pollut. 2007, 183, 165–176. [Google Scholar] [CrossRef]
- Boer, C.G.; Obici, L.; Souza, G.M.; Peralta, R.M. Decolorization of synthetic dyes by solid state cultures of Lentinula (Lentinus) edodes producing manganese peroxidase as the main ligninolytic enzyme. Bioresour. Technol. 2004, 94, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, H.M.N.; Asgher, M.; Bhatti, H.N. Optimization of physical and nutritional factors for synthesis of lignin degrading enzymes by a novel strain of Trametes versicolor. BioResources 2011, 6, 1273–1287. [Google Scholar]
- Apprich, S.; Tirpanalan, O.; Hell, J.; Reisinger, M.; Bohmdorfer, S.; Siebenhandl-Ehm, S.; Novalin, S.; Kneifel, W. Wheat bran-based biorefinery 2: Valorization of products. Food Sci. Technol. 2014, 56, 222–231. [Google Scholar] [CrossRef]
- Sharma, R.K.; Arora, D.S. Production of lignocellulolytic enzymes and enhancement of in vitro digestibility during solid state fermentation of wheat straw by Phlebia floridensis. Bioresour. Technol. 2010, 101, 9248–9253. [Google Scholar] [CrossRef] [PubMed]
- Ma, L.; Cui, Y.; Cai, R.; Liu, X.; Zhang, C.; Xiao, D. Optimization and evaluation of alkaline potassium permanganate pretreatment of corncob. Bioresour. Technol. 2015, 180, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Sathishkumar, P.; Murugesan, K.; Palvannan, T. Production of laccase from Pleurotus florida using agro-wastes and efficient decolorization of Reactive blue 198. J. Basic Microb. 2010, 50, 360–367. [Google Scholar] [CrossRef] [PubMed]
- Iandolo, D.; Piscitelli, A.; Sannia, G.; Faraco, V. Enzyme Production by Solid Substrate Fermentation of Pleurotus ostreatus and Trametes versicolor on Tomato Pomace. Appl. Biochem. Biotechnol. 2011, 163, 40–51. [Google Scholar] [CrossRef] [PubMed]
- Garcia, T.A.; Santiago, M.F.; Ulhoa, C.J. Studies on the Pycnoporus sanguineus CCT-4518 laccase purified by hydrophobic interaction chromatography. Appl. Microbiol. Biotechnol. 2007, 75, 311–318. [Google Scholar] [CrossRef] [PubMed]
- Litthauer, D.; Vuuren, M.J.V.; Tonder, A.V.; Wolfaardt, R.W. Purification and kinetics of a thermostable laccase from Pycnoporus sanguineus (SCC 108). Enzym. Microb. Technol. 2007, 40, 563–568. [Google Scholar] [CrossRef]
- Trovaslet, M.; Enaud, E.; Guiavarc’h, Y.; Corbisier, A.M.; Vanhulle, S. Potential of a Pycnoporus sanguineus laccase in bioremediation of wastewater and kinetic activation in the presence of an anthraquinonic acid dye. Enzym. Microb. Technol. 2007, 41, 368–376. [Google Scholar] [CrossRef]
- Dantán-González, E.; Vite-Vallejo, E.O.; Martínez-Anaya, C.; Méndez-Sánchez, M.; González, M.C.; Palomares, L.A.; Folch-Mallol, J. Production of two novel laccase isoforms by a thermotolerant strain of Pycnoporus sanguineus isolated from an oil-polluted tropical habitat. Int. Microbiol. 2008, 11, 163–169. [Google Scholar] [PubMed]
- Vite-Vallejo, O.; Palomares, L.A.; Dantán-González, E.; Ayala-Castro, H.G.; Martínez-Anaya, C.; Valderrama, B.; Folch-Mallol, J. The role of N-glycosylation on the enzymatic activity of a Pycnoporus sanguineus Laccase. Enzym. Microb. Technol. 2009, 45, 233–239. [Google Scholar] [CrossRef]
- Hoshida, H.; Nakao, M.; Kanazawa, H.; Kubo, K.; Hakukawa, T.; Morimasa, K.; Akada, R.; Nishizawa, Y. Isolation of Five Laccase Gene Sequence from the White-Rot Fungus Trametes sanguinea by PCR, and Cloning, Characterization and Expression of the Laccase cDNA in Yeasts. J. Biosci. Bioeng. 2001, 92, 372–380. [Google Scholar] [CrossRef]
- Pointing, S.B.; Jones, E.B.G.; Vrijmoed, L.L.P. Optimization of laccase production by Pycnoporus sanguineus in submerged liquid culture. Mycologia 2000, 92, 139–144. [Google Scholar] [CrossRef]
- Ding, Z.; Chen, Y.; Xu, Z.; Peng, L.; Xu, G.; Gu, Z.; Zhang, L.; Shi, G.; Zhang, K. Production and characterization of laccase from Pleurotus ferulae in submerged fermentation. Ann. Microbiol. 2014, 64, 121–129. [Google Scholar] [CrossRef]
- More, S.S.; Renuka, P.S.; Pruthvi, K.; Swetha, M.; Malini, S.; Veena, S.M. Isolation, Purification, and Characterization of Fungal Laccase from Pleurotus sp. Enzym. Res. 2011, 2011, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Ding, Z.; Peng, L.; Chen, Y.; Zhang, L.; Gu, Z.; Shi, G.; Zhang, K. Production and characterization of thermostable laccase from the mushroom, Ganoderma lucidum, using submerged fermentation. Afr. J. Microbiol. Res. 2012, 6, 1147–1157. [Google Scholar]
- Galhaup, C.; Goller, S.; Peterbauer, C.K.; Strauss, J.; Haltrich, D. Characterization of the major laccase isoenzyme from Trametes pubescens and regulation of its synthesis by metal ions. Microbiology 2002, 148, 2159–2169. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Gupte, S.; Gahlout, M.; Gupte, A. Purification and characterization of an extracelular laccase from solid-state culture of Pleurotus ostreatus HP-1. 3 Biotech 2014, 4, 77–84. [Google Scholar] [CrossRef]
- Si, J.; Peng, F.; Cui, B. Purification, biochemical characterization and dye decolorization capacity of an alkali-resistant and metal-tolerant laccase from Trametes pubescens. Bioresour. Technol. 2013, 128, 49–57. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Chen, D.; Yang, E.; Niu, J.; Chen, Y.; Chagan, I. Purification and characterization of a thermotolerant laccase isoform in Trametes trogii strain and its potential in dye decolorization. Int. Biodeter. Biodegr. 2014, 93, 186–194. [Google Scholar] [CrossRef]
- Asgher, M.; Hafiz, M.; Iqbal, N.; Asad, M.J. Kinetic characterization of purified laccase produced from Trametes versicolor IBL-04 in solid state-Bio-Processing of corncobs. BioResources 2012, 7, 1171–1188. [Google Scholar]
- Aquino Neto, S.; Zimbardi, A.L.R.L.; Cardoso, F.P.; Crepaldi, L.B.; Minteer, S.D.; Jorge, J.A.; Furriel, R.P.M.; de Andrade, A.R. Potential application of laccase from Pycnoporus sanguineus in methanol/O2 biofuel cells. J. Electroanal. Chem. 2016, 765, 2–7. [Google Scholar] [CrossRef]
- Xiao, Y.Z.; Tu, X.M.; Wang, J.; Zhang, M.; Cheng, Q.; Zeng, W.Y.; Shi, Y.Y. Purification, molecular characterization and reactivity with aromatic compounds of a laccase from basidiomycete Trametes sp. strain AH28–2. Appl. Microbiol. Biotechnol. 2003, 60, 700–707. [Google Scholar] [CrossRef] [PubMed]
- Chairin, T.; Nitheranont, T.; Watanabe, A.; Asada, Y.; Khanongnuch, C.; Lumyong, S. Purification and characterization of the extracellular laccase produced by Trametes polyzona WR710-1 under solid-state fermentation. J. Basic Microb. 2014, 54, 35–43. [Google Scholar] [CrossRef] [PubMed]
- Nagai, M.; Sato, T.; Watanabe, H.; Saito, K. Purification and characterization of an extracellular laccase from the edible mushroom Lentinula edodes, and decolorization of chemically different dyes. Appl. Microbiol. Biotechnol. 2002, 60, 327–335. [Google Scholar] [PubMed]
- Morozova, O.V.; Shumakovich, G.P.; Gorbacheva, M.A.; Shleev, S.V.; Yaropolov, A.I. “Blue” Laccases. Biochemistry 2007, 72, 1136–1150. [Google Scholar] [CrossRef] [PubMed]
- Shleev, S.V.; Morozova, O.V.; Nikitina, O.V.; Gorshina, E.S.; Rusinova, T.V.; Serezhenkov, V.S.; Burbaev, D.S.; Gazaryan, I.G.; Yaropolov, A.I. Comparison of physico-chemical characterisitcs of four laccases from different basidiomycetes. Biochimie 2004, 86, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Azmi, W.; Sani, R.K.; Banerjee, U.C. Biodegradation of triphenylmethane dyes. Enzym. Microb. Technol. 1998, 22, 185–191. [Google Scholar] [CrossRef]
- Forootanfar, H.; Moezzi, A.; Aghaie-Khozini, M.; Mahmoudjanlou, Y.; Ameri, A.; Niknejad, F.; Faramarzi, M.A. Synthetic dye decolorization by three sources of fungal laccase. Iran. J. Environ. Health Sci. Eng. 2012, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Grassi, E.; Scodeller, P.; Filiel, N.; Carballo, R.; Levin, L. Potential of Trametes trogii culture fluids and its purified laccase for the decolorization of different types of recalcitrant dyes without the addition of redox mediators. Int. Biodeter. Biodegr. 2011, 65, 635–643. [Google Scholar] [CrossRef]
- Rodríguez-Couto, S. Production of laccase and decolouration of the textile dye Remazol Brilliant Blue R in temporary immersion bioreactors. J. Hazard. Mater. 2011, 194, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, M.; Ray, L. Adsorption behavior of Reactive Blue 4, a tri-azine dye on dry cells of Rhizopus oryzae in a batch system. Chem. Spec. Bioavailab. 2015, 27, 112–120. [Google Scholar] [CrossRef]
- Zeng, X.; Cai, Y.; Liao, X.; Zeng, X.; Li, W.; Zhang, D. Decolorization of synthetic dyes by crude laccase from a newly isolated Trametes trogii strain cultivated on solid agro-industrial residue. J. Hazard. Mater. 2011, 187, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.Q.; Zhao, X.X.; Liu, C.Y.; Zheng, Y.; Qian, S.J. Decolorization of azo, triphenylmethane and anthraquinone dyes by a newly isolated Trametes sp. SQ01 and its laccase. Process Biochem. 2009, 44, 1185–1189. [Google Scholar] [CrossRef]
- Yang, J.; Lin, Q.; Ng, T.B.; Ye, X.; Lin, J. Purification and Characterization of a Novel Laccase from Cerrena sp. HYB07 with Dye Decolorizing Ability. PLoS ONE 2014, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Hadibarata, T.; Yusoff, A.R.M.; Aris, A.; Samiati; Hidayat, T.; Kristanti, R.A. Decolorization of Azo, Triphenylmethane and Anthraquinone Dyes by Laccase of a Newly Isolated Armillaria sp. F022. Water Air Soil Pollut. 2012, 223, 1045–1054. [Google Scholar] [CrossRef]
- Deswal, D.; Sharma, A.; Gupta, R.; Kujad, R.C. Application of lignocellulolytic enzymes produced under solid state cultivation conditions. Bioresour. Technol. 2012, 115, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, R.; Ma, L.; Fan, F.; Gong, Y.; Wan, X.; Jiang, M.; Zhang, X.; Yang, Y. Decolorization of different dyes by a newly isolated white-rot fungi strain Ganoderma sp. En3 and cloning and functional analysis of its laccase gene. J. Hazard. Mater. 2011, 192, 855–873. [Google Scholar] [CrossRef] [PubMed]
- Osma, J.F.; Toca-Herrera, J.L.; Rodríguez-Couto, S. Transformation pathway of Remazol Brilliant Blue R by immobilised laccase. Bioresour. Technol. 2010, 101, 8509–8514. [Google Scholar] [CrossRef] [PubMed]
- Rezaei, S.; Tahmasbi, H.; Mogharabi, M.; Firuzyar, S.; Ameri, A.; Khoshayand, M.R.; Faramarzi, M.A. Efficient decolorization and detoxification of reactive orange 7 using laccase isolated from Paraconiothyrium variable, kinetics and energetics. J. Taiwan Inst. Chem. Eng. 2015, 56, 113–121. [Google Scholar] [CrossRef]
- Ryvarden, L. Genera of Polypores: Nomenclature and Taxonomy. Synopsis Fungorum 1991, 5, 1–363. [Google Scholar]
- Teixeira, A.R. Chave para identificação dos gêneros de Polyporaceae com base na morfologia do basidiocarpo. Boletim do Instituto de Botânica 1993, 8, 1–55. [Google Scholar]
- Teixeira, A.R. Método para estudo das hifas do basidiocarpo de fungos poliporáceos. Manual do Instituto de Botânica 1995, 6, 1–20. [Google Scholar]
- McIlvaine, T.C. A buffer solution for colorimetric comparison. J. Biol. Chem. 1921, 49, 183–186. [Google Scholar]
- Read, S.M.; Northcote, D.H. Minimization of variation in the response to different protein of the Coomassie blue G dye-binding assay for protein. Anal. Biochem. 1981, 116, 53–64. [Google Scholar] [CrossRef]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Colorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 2, 350–356. [Google Scholar] [CrossRef]
- Davis, B.J. Disc electrophoresis-II Method and Application to Human Serum Proteins. Ann. N. Y. Acad. Sci. 1964, 121, 404–427. [Google Scholar] [CrossRef] [PubMed]
- Laemmli, U.K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 1970, 227, 680–685. [Google Scholar] [CrossRef] [PubMed]
- Souza, F.H.M.; Nascimento, C.V.; Rosa, J.C.; Masui, D.C.; Leone, F.A.; Jorge, J.A.; Furriel, R.P.M. Purification and biochemical characterization of a mycelial glucose- and xylose-stimulated-β-glucosidase from the thermophilic fungus Humicola insolens. Process Biochem. 2010, 45, 272–278. [Google Scholar] [CrossRef]
- Leone, F.A.; Baranauskas, J.A.; Furriel, R.P.M.; Borin, I.A. SigrafW: An easy-to-use program for fitting enzyme kinetic data. Biochem. Mol. Biol. Educ. 2005, 33, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Shin, W.; Brown, S.H.; Wahleithner, J.A.; Sundaram, U.M.; Solomon, E.I. A study of a series of recombinant fungal laccases and bilirubin oxidase that exhibit significant differences in redox potential, substrate specificity, and stability. Biochim. Biophys. Acta 1996, 1292, 303–311. [Google Scholar] [CrossRef]
- Vanysek, P. Handbook of Chemistry and Physics, 73rd ed.; CRC Press: Boca Raton, FL, USA, 1992; pp. 8:17–8:22. [Google Scholar]





| Carbon Source | U·g−1 | U·mL−1 | |
|---|---|---|---|
| Wheat bran | 29.8 ± 1.9 | 5.9 ± 0.4 | |
| Steam-exploded sugarcane bagasse | ND a | ND | |
| Raw sugarcane bagasse | ND | ND | |
| Peanut hull | 2.1 ± 0.2 | 0.40 ± 0.03 | |
| Rice husk | 1.9 ± 0.1 | 0.40 ± 0.02 | |
| Milled corncob | ND | ND | |
| Milled Soybean | ND | ND | |
| Sugarcane trash | ND | ND | |
| Carbon Supplement (1% w/w) | U·g−1 | % | |
| None | 30.1 ± 1.8 | 100.0 | |
| Peanut hull | 31.9 ± 1.4 | 105.8 | |
| Rice husk | 29.6 ± 1.1 | 98.2 | |
| Soybean meal | 23.7 ± 1.1 | 78.7 | |
| Corn husk | 25.5 ± 1.3 | 84.6 | |
| Sugarcane trash | 30.5 ± 1.7 | 101.3 | |
| Glucose | 28.2 ± 1.1 | 93.7 | |
| Milled corncob | 35.5 ± 1.7 | 117.8 | |
| Nitrogen Supplement | % (w/w) | U·g−1 | % |
| None | -- | 33.5 ± 1.7 | 100.0 |
| Asparagine | 1 | 35.3 ± 1.5 | 105.6 |
| Casein | 1 | 37.3 ± 1.6 | 111.2 |
| Soybean meal | 1 | 26.9 ± 1.2 | 80.4 |
| Glycine | 1 | 29.2 ± 1.3 | 87.4 |
| Peptone | 1 | 46.0 ± 2.4 | 137.4 |
| Yeast extract | 1 | 35.8 ± 1.9 | 107.0 |
| Malt extract | 1 | 39.0 ± 1.9 | 116.4 |
| NH4NO3 | 0.8 | 31.0 ± 1.7 | 92.8 |
| KNO3 | 0.8 | 33.6 ± 1.5 | 100.5 |
| NaNO3 | 0.8 | 17.6 ± 0.9 | 52.2 |
| (NH4)2SO4 | 0.8 | 29.4 ± 1.3 | 87.7 |
| NH4Cl | 0.8 | 56.1 ± 2.7 | 167.8 |
| Urea | 0.8 | 35.9 ± 2.0 | 107.3 |
| Run | Real (Coded) Values | Laccase (U·g−1) | |||
|---|---|---|---|---|---|
| Time | Initial Moisture | Temperature | Milled Corncob | ||
| (Days) | (mL·g−1) | (°C) | (%, w/w) | ||
| 1 | 7 (−1) | 3 (−1) | 20 (−1) | 10 (−1) | 47.5 ± 3.9 |
| 2 | 9 (+1) | 3 (−1) | 20 (−1) | 10 (−1) | 56.7 ± 4.1 |
| 3 | 7 (−1) | 5 (+1) | 20 (−1) | 10 (−1) | 36.9 ± 5.1 |
| 4 | 9 (+1) | 5 (+1) | 20 (−1) | 10 (−1) | 42.9 ± 4.3 |
| 5 | 7 (−1) | 3 (−1) | 30 (+1) | 10 (−1) | 49.1 ± 5.7 |
| 6 | 9 (+1) | 3 (−1) | 30 (+1) | 10 (−1) | 47.6 ± 5.3 |
| 7 | 7 (−1) | 5 (+1) | 30 (+1) | 10 (−1) | 68.2 ± 6.1 |
| 8 | 9 (+1) | 5 (+1) | 30 (+1) | 10 (−1) | 59.7 ± 6.3 |
| 9 | 7 (−1) | 3 (−1) | 20 (−1) | 20 (+1) | 73.9 ± 6.9 |
| 10 | 9 (+1) | 3 (−1) | 20 (−1) | 20 (+1) | 77.2 ± 7.4 |
| 11 | 7 (−1) | 5 (+1) | 20 (−1) | 20 (+1) | 45.7 ± 3.9 |
| 12 | 9 (+1) | 5 (+1) | 20 (−1) | 20 (+1) | 48.6 ± 4.6 |
| 13 | 7 (−1) | 3 (−1) | 30 (+1) | 20 (+1) | 65.7 ± 5.9 |
| 14 | 9 (−1) | 3 (−1) | 30 (+1) | 20 (+1) | 74.7 ± 8.4 |
| 15 | 7 (−1) | 5 (+1) | 30 (+1) | 20 (+1) | 112.9 ± 10.9 |
| 16 | 9 (+1) | 5 (+1) | 30 (+1) | 20 (+1) | 95.1 ± 9.7 |
| 17 | 6 (−2) | 4 (0) | 25 (0) | 15 (0) | 115.2 ± 10.6 |
| 18 | 10 (+2) | 4 (0) | 25 (0) | 15 (0) | 119.8 ± 12.3 |
| 19 | 8 (0) | 2 (−2) | 25 (0) | 15 (0) | 33.1 ± 2.1 |
| 20 | 8 (0) | 6 (+2) | 25 (0) | 15 (0) | 78.3 ± 6.4 |
| 21 | 8 (0) | 4 (0) | 15 (−2) | 15 (0) | 29.4 ± 2.4 |
| 22 | 8 (0) | 4 (0) | 35 (+2) | 15 (0) | 54.6 ± 4.8 |
| 23 | 8 (0) | 4 (0) | 25 (0) | 5 (−2) | 51.6 ± 4.1 |
| 24 | 8 (0) | 4 (0) | 25 (0) | 25 (+2) | 119.5 ± 12.5 |
| 25 | 8 (0) | 4 (0) | 25 (0) | 15 (0) | 136.4 ± 14.7 |
| 26 | 8 (0) | 4 (0) | 25 (0) | 15 (0) | 137.2 ± 14.2 |
| 27 | 8 (0) | 4 (0) | 25 (0) | 15 (0) | 135.8 ± 13.1 |
| Effect | Coefficient | p-Value | |||
|---|---|---|---|---|---|
| Intercept | 124.33 | 0.000000 | |||
| (x) Time (Q) | −12.54 | 0.001921 | |||
| (y) Initial Moisture (Q) | −23.42 | 0.000002 | |||
| (z) Temperature (L) | 9.72 | 0.011855 | |||
| (z) Temperature (Q) | −20.04 | 0.000014 | |||
| (w) Milled Corncob (L) | 8.10 | 0.031957 | |||
| Zy | 11.25 | 0.016584 | |||
| Source | SS a | Df b | MSq c | Fcalc | |
| Regression | 24,581.59 | 6 | 4096.93 | 13.83 | |
| Residual | 5923.10 | 20 | 296.15 | – | |
| Total | 30,504.69 | 26 | 1173.26 | – | |
| Step | Total Activity (U) | Total Protein (mg) | Specific Activity (U·mg−1) | Yield (%) | Purification (Fold) |
|---|---|---|---|---|---|
| Crude extract | 548.0 | 32.70 | 16.8 | 100 | 1 |
| 60% (NH4)2SO4 | 344.7 | 13.10 | 26.3 | 63 | 1.6 |
| Phenyl-Sepharose | 164.4 | 0.40 | 411.0 | 30 | 24.5 |
| Run | Real (Coded) Values | U·mg−1 | |
|---|---|---|---|
| T (°C) | pH | ||
| 1 | 70 (−1) | 4.0 (−1) | 258.4 ± 11.9 |
| 2 | 80 (+1) | 4.0 (−1) | 176.8 ± 8.5 |
| 3 | 70 (−1) | 5.0 (+1) | 123.6 ± 6.9 |
| 4 | 80 (+1) | 5.0 (+1) | 114.9 ± 7.8 |
| 5 | 75 (0) | 3.5 (−1.41) | 193.8 ± 9.7 |
| 6 | 75 (0) | 5.5 (+1.41) | 91.4 ± 4.7 |
| 7 | 68 (−1.41) | 4.5 (0) | 248.0 ± 12.4 |
| 8 | 82 (+1.41) | 4.5 (0) | 197.9 ± 9.9 |
| 9 | 75 (0) | 4.5 (0) | 396.1 ± 19.8 |
| 10 | 75 (0) | 4.5 (0) | 395.4 ± 19.5 |
| 11 | 75 (0) | 4.5 (0) | 397.1 ± 20.8 |
| Effects | Coefficient | p-value | |||
|---|---|---|---|---|---|
| Intercept | 396.25 | 0.0000002 | |||
| pH (L) | −47.99 | 0.000039 | |||
| pH (Q) | −135.57 | 0.000007 | |||
| T (L) | −20.24 | 0.000224 | |||
| T(Q) | −89.72 | 0.000016 | |||
| pH.T | 18.23 | 0.000546 | |||
| Source | SS a | Df b | MSq c | F | |
| Regression | 141,950.64 | 5 | 28,390.12 | – | |
| Residual | 106.22 | 5 | 21.24 | 1336.63 | |
| Total | 142,056.81 | 10 | 14,205.68 | – | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zimbardi, A.L.R.L.; Camargo, P.F.; Carli, S.; Aquino Neto, S.; Meleiro, L.P.; Rosa, J.C.; De Andrade, A.R.; Jorge, J.A.; Furriel, R.P.M. A High Redox Potential Laccase from Pycnoporus sanguineus RP15: Potential Application for Dye Decolorization. Int. J. Mol. Sci. 2016, 17, 672. https://doi.org/10.3390/ijms17050672
Zimbardi ALRL, Camargo PF, Carli S, Aquino Neto S, Meleiro LP, Rosa JC, De Andrade AR, Jorge JA, Furriel RPM. A High Redox Potential Laccase from Pycnoporus sanguineus RP15: Potential Application for Dye Decolorization. International Journal of Molecular Sciences. 2016; 17(5):672. https://doi.org/10.3390/ijms17050672
Chicago/Turabian StyleZimbardi, Ana L. R. L., Priscila F. Camargo, Sibeli Carli, Sidney Aquino Neto, Luana P. Meleiro, Jose C. Rosa, Adalgisa R. De Andrade, João A. Jorge, and Rosa P. M. Furriel. 2016. "A High Redox Potential Laccase from Pycnoporus sanguineus RP15: Potential Application for Dye Decolorization" International Journal of Molecular Sciences 17, no. 5: 672. https://doi.org/10.3390/ijms17050672
APA StyleZimbardi, A. L. R. L., Camargo, P. F., Carli, S., Aquino Neto, S., Meleiro, L. P., Rosa, J. C., De Andrade, A. R., Jorge, J. A., & Furriel, R. P. M. (2016). A High Redox Potential Laccase from Pycnoporus sanguineus RP15: Potential Application for Dye Decolorization. International Journal of Molecular Sciences, 17(5), 672. https://doi.org/10.3390/ijms17050672