Spatial Geometries of Self-Assembled Chitohexaose Monolayers Regulate Myoblast Fusion
Abstract
:1. Introduction
2. Results
2.1. Effects of Topographical Features and Chitooligomers on Myotube Formation
2.2. Hexa-N-acetyl-d-glucosamine (GlcNAc6)-self-assembled Monolayers (SAMs) Regulate Glucose Transporter Type 4 (GLUT4) mRNA Expression
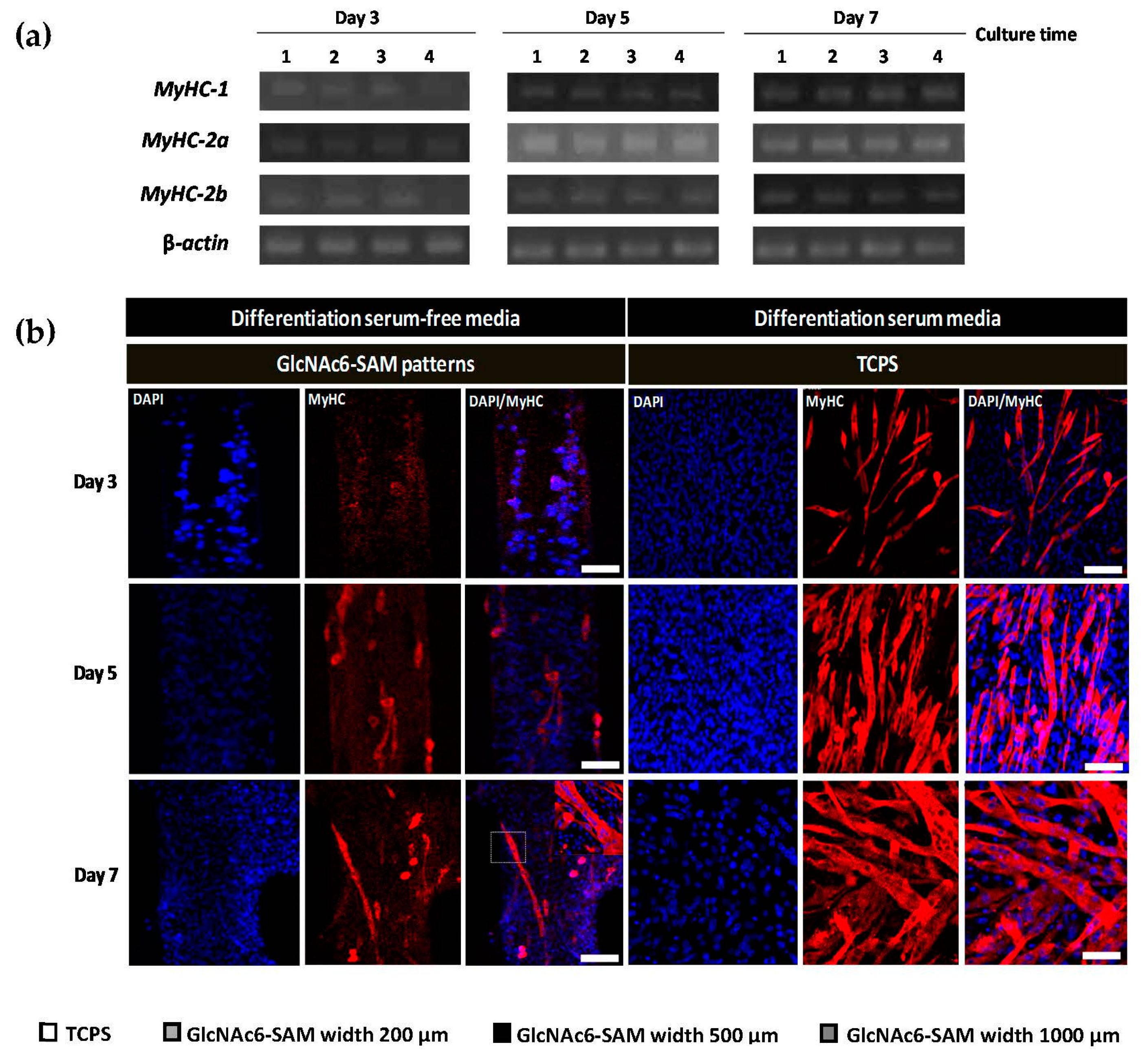
2.3. Early and Late Stages of Myoblast Fusion on GlcNAc6-SAM Patterns
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Synthesis and Fabrication of Carbohydrate-Functionalized Monolayers on Gold Micropatterns
4.3. Cell Culture Assay
4.4. Quantification of mRNA by Real-Time Quantitative Polymerase Chain Reaction (RT-PCR)
4.5. Glucose Uptake Assay
4.6. F-Actin Staining
4.7. Immunostaining of MyHC
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hindi, S.M.; Tajrishi, M.M.; Kumar, A. Signaling mechanisms in mammalian myoblast fusion. Sci. Signal. 2013, 6, re2. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Jin, P.; Duan, R.; Chen, E.H. Mechanisms of myoblast fusion during muscle development. Curr. Opin. Genet. Dev. 2015, 32, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.; Li, E.; Nie, Q.; Zhang, X. Myomaker, regulated by MYOD, MYOG and miR-140-3P, promotes chicken myoblast fusion. Int. J. Mol. Sci. 2015, 16, 26186–26201. [Google Scholar] [CrossRef] [PubMed]
- Rochlin, K.; Yu, S.; Roy, S.; Baylies, M.K. Myoblast fusion: When it takes more to make one. Dev. Biol. 2010, 341, 66–83. [Google Scholar] [CrossRef] [PubMed]
- Stern-Straeter, J.; Bran, G.; Riedel, F.; Sauter, A.; Hörmann, K.; Goessler, U.R. Characterization of human myoblast cultures for tissue engineering. Int. J. Mol. Med. 2008, 21, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Stern-Straeter, J.; Riedel, F.; Bran, G.; Hörmann, K.; Goessler, U.R. Advances in skeletal muscle tissue engineering. In Vivo 2007, 21, 435–444. [Google Scholar] [PubMed]
- Stevenson, E.J.; Koncarevic, A.; Giresi, P.G.; Jackman, R.W.; Kandarian, S.C. Transcriptional profile of a myotube starvation model of atrophy. J. Appl. Physiol. 2005, 98, 1396–1406. [Google Scholar] [CrossRef] [PubMed]
- Franke, J.; Abs, V.; Zizzadoro, C.; Abraham, G. Comparative study of the effects of fetal bovine serum versus horse serum on growth and differentiation of primary equine bronchial fibroblasts. BMC Vet. Res. 2014, 10, 119. [Google Scholar] [CrossRef] [PubMed]
- Das, M.; Rumsey, J.W.; Bhargava, N.; Stancescu, M.; Hickman, J.J. Skeletal muscle tissue engineering: A maturation model promoting long-term survival of myotubes, structural development of the excitation-contraction coupling apparatus and neonatal myosin heavy chain expression. Biomaterials 2009, 30, 5392–5402. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, E.J.; Giresi, P.G.; Koncarevic, A.; Kandarian, S.C. Global analysis of gene expression patterns during disuse atrophy in rat skeletal muscle. J. Physiol. 2003, 551, 33–48. [Google Scholar] [CrossRef] [PubMed]
- Laurin, M.; Côté, J. Insights into the biological functions of Dock family guanine nucleotide exchange factors. Genes Dev. 2014, 28, 533–547. [Google Scholar] [CrossRef] [PubMed]
- Aguilar, P.S.; Baylies, M.K.; Fleissner, A.; Helming, L.; Inoue, N.; Podbilewicz, B.; Wang, H.; Wong, M. Genetic basis of cell-cell fusion mechanisms. Trends Genet. 2013, 29, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Braun, T.; Gautel, M. Transcriptional mechanisms regulating skeletal muscle differentiation, growth and homeostasis. Nat. Rev. Mol. Cell Biol. 2011, 12, 349–361. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Shi, X.E.; Song, C.; Sun, S.; Yang, G.; Li, X. BAMBI promotes C2C12 myogenic differentiation by enhancing Wnt/β-catenin signaling. Int. J. Mol. Sci. 2015, 16, 17734–17745. [Google Scholar] [CrossRef] [PubMed]
- Lam, M.T.; Sim, S.; Zhu, X.; Takayama, S. The effect of continuous wavy micropatterns on silicone substrates on the alignment of skeletal muscle myoblasts and myotubes. Biomaterials 2006, 27, 4340–4347. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, V.; Ahadian, S.; Ostrovidov, S.; Camci-Unal, G.; Chen, S.; Kaji, H.; Ramalingam, M.; Khademhosseini, A. Engineered contractile skeletal muscle tissue on a microgrooved methacrylated gelatin substrate. Tissue Eng. Part A 2012, 18, 2453–2465. [Google Scholar] [CrossRef] [PubMed]
- Engler, A.J.; Griffin, M.A.; Sen, S.; Bönnemann, C.G.; Sweeney, H.L.; Discher, D.E. Myotubes differentiate optimally on substrates with tissue-like stiffness: Pathological implications for soft or stiff microenvironments. J. Cell Biol. 2004, 166, 877–887. [Google Scholar] [CrossRef] [PubMed]
- Boontheekul, T.; Hill, E.E.; Kong, H.J.; Mooney, D.J. Regulating myoblast phenotype through controlled gel stiffness and degradation. Tissue Eng. 2007, 13, 1431–1442. [Google Scholar] [CrossRef] [PubMed]
- Zatti, S.; Zoso, A.; Serena, E.; Luni, C.; Cimetta, E.; Elvassore, N. Micropatterning topology on soft substrates affects myoblast proliferation and differentiation. Langmuir 2012, 28, 2718–2726. [Google Scholar] [CrossRef] [PubMed]
- Huang, N.F.; Patel, S.; Thakar, R.G.; Wu, J.; Hsiao, B.S.; Chu, B.; Lee, R.J.; Li, S. Myotube assembly on nanofibrous and micropatterned polymers. Nano Lett. 2006, 6, 537–542. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.H.; Shi, X.; Zaia, J.; Ai, X. Heparan sulfate 6-O-endosulfatases (Sulfs) coordinate the Wnt signaling pathways to regulate myoblast fusion during skeletal muscle regeneration. J. Biol. Chem. 2012, 287, 32651–32664. [Google Scholar] [CrossRef] [PubMed]
- Yokota, S.; Kitaoka, T.; Wariishi, H. Biofunctionality of self-assembled nanolayers composed of cellulosic polymers. Carbohydr. Polym. 2008, 74, 666–672. [Google Scholar] [CrossRef]
- Tanaka, N.; Yoshiike, Y.; Yoshiyama, C.; Kitaoka, T. Self-assembly immobilization of hyaluronan thiosemicarbazone on a gold surface for cell culture applications. Carbohydr. Polym. 2010, 82, 100–105. [Google Scholar] [CrossRef]
- Yoshiike, Y.; Kitaoka, T. Tailoring hybrid glyco-nanolayers composed of chitohexaose and cellohexaose for cell culture applications. J. Mater. Chem. 2011, 21, 11150–11158. [Google Scholar] [CrossRef]
- Poosala, P.; Kitaoka, T. Chitooligomer-immobilized biointerfaces with micropatterned geometries for unidirectional alignment of myoblast cells. Biomolecules 2016, 6, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tortorella, L.L.; Pilch, P.F. C2C12 myocytes lack an insulin-responsive vesicular compartment despite dexamethasone-induced GLUT4 expression. Am. J. Physiol. Endocrinol. Metab. 2002, 283, E514–E524. [Google Scholar] [CrossRef] [PubMed]
- Derave, W.; Ai, H.; Ihlemann, J.; Witters, L.A.; Kristiansen, S.; Richter, E.A.; Ploug, T. Dissociation of AMP-activated protein kinase slow-twitch muscle. Diabetes 2000, 49, 1281–1287. [Google Scholar] [CrossRef] [PubMed]
- Sandström, M.E.; Zhang, S.J.; Westerblad, H.; Katz, A. Mechanical load plays little role in contraction-mediated glucose transport in mouse skeletal muscle. J. Physiol. 2007, 579, 527–534. [Google Scholar] [CrossRef] [PubMed]
- Sakiyama, K.; Abe, S.; Tamatsu, Y.; Ide, Y.; Akiyama, K.S.; Be, S.A.; Amatsu, Y.T.; De, Y.I. Effects of stretching stress on the muscle contraction proteins of skeletal muscle myoblasts. Biomed. Res. 2005, 26, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Manabe, Y.; Miyatake, S.; Takagi, M.; Nakamura, M.; Okeda, A.; Nakano, T.; Hirshman, M.F.; Goodyear, L.J.; Fujii, N.L. Characterization of an acute muscle contraction model using cultured C2C12 myotubes. PLoS ONE 2012, 7, e52592. [Google Scholar] [CrossRef] [PubMed]
- Michael, L.F.; Wu, Z.; Cheatham, R.B.; Puigserver, P.; Adelmant, G.; Lehman, J.J.; Kelly, D.P.; Spiegelman, B.M. Restoration of insulin-sensitive glucose transporter (GLUT4) gene expression in muscle cells by the transcriptional coactivator PGC-1. Proc. Natl. Acad. Sci. USA 2001, 98, 3820–3825. [Google Scholar] [CrossRef] [PubMed]
- Leney, S.E.; Tavaré, J.M. The molecular basis of insulin-stimulated glucose uptake: Signalling, trafficking and potential drug targets. J. Endocrinol. 2009, 203, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Leto, D.; Saltiel, A.R. Regulation of glucose transport by insulin: Traffic control of GLUT4. Nat. Rev. Mol. Cell Biol. 2012, 13, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Torgan, C.E.; Daniels, M.P. Regulation of myosin heavy chain expression during rat skeletal muscle development in vitro. Mol. Biol. Cell 2001, 12, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Staron, R.S.; Johnson, P. Myosin polymorphism and differential expression in adult human skeletal muscle. Comp. Biochem. Physiol. B 1993, 106, 463–475. [Google Scholar] [CrossRef]
- Allen, D.L.; Leinwand, L.A. Postnatal myosin heavy chain isoform expression in normal mice and mice null for IIb or IId myosin heavy chains. Dev. Biol. 2001, 229, 383–395. [Google Scholar] [CrossRef] [PubMed]
- Lu, B.D.; Allen, D.L.; Leinwand, L.A.; Lyons, G.E. Spatial and temporal changes in myosin heavy chain gene expression in skeletal muscle development. Dev. Biol. 1999, 216, 312–326. [Google Scholar] [CrossRef] [PubMed]
- Millay, D.P.; O’Rourke, J.R.; Sutherland, L.B.; Bezprozvannaya, S.; Shelton, J.M.; Bassel-Duby, R.; Olson, E.N. Myomaker is a membrane activator of myoblast fusion and muscle formation. Nature 2013, 499, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Gruenbaum-Cohen, Y.; Harel, I.; Umansky, K.B.; Tzahor, E.; Snapper, S.B.; Shilo, B.Z.; Schejter, E.D. The actin regulator N-WASp is required for muscle-cell fusion in mice. Proc. Natl. Acad. Sci. USA 2012, 109, 11211–11216. [Google Scholar] [CrossRef] [PubMed]
- Nowak, S.J.; Nahirney, P.C.; Hadjantonakis, A.K.; Baylies, M.K. Nap1-mediated actin remodeling is essential for mammalian myoblast fusion. J. Cell Sci. 2009, 122, 3282–3293. [Google Scholar] [CrossRef] [PubMed]
- Meriane, M.; Roux, P.; Primig, M.; Fort, P.; Gauthier-Rouvière, C. Critical activities of Rac1 and Cdc42Hs in skeletal myogenesis: Antagonistic effects of JNK and p38 pathways. Mol. Biol. Cell 2000, 11, 2513–2528. [Google Scholar] [CrossRef] [PubMed]
- Hakeda-Suzuki, S.; Ng, J.; Tzu, J.; Dietzl, G.; Sun, Y.; Harms, M.; Nardine, T.; Luo, L.; Dickson, B.J. Rac function and regulation during Drosophila development. Nature 2002, 416, 438–442. [Google Scholar] [CrossRef] [PubMed]
- Castellani, L.; Salvati, E.; Alemà, S.; Falcone, G. Fine regulation of RhoA and Rock is required for skeletal muscle differentiation. J. Biol. Chem. 2006, 281, 15249–15257. [Google Scholar] [CrossRef] [PubMed]
- Charrasse, S.; Comunale, F.; Fortier, M.; Portales-Casamar, E.; Debant, A.; Gauthier-Rouvière, C. M-cadherin activates Rac1 GTPase through the Rho-GEF Trio during myoblast fusion. Mol. Biol. Cell 2007, 18, 1734–1743. [Google Scholar] [CrossRef] [PubMed]
- Duan, R.; Gallagher, P.J. Dependence of myoblast fusion on a cortical actin wall and nonmuscle myosin IIA. Dev. Biol. 2009, 325, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Huard, J.; Li, Y.; Fu, F.H. Muscle injuries and repair: Current trends in research. J. Bone Jt. Surg. Am. 2002, 84-A, 822–832. [Google Scholar]
- Swailes, N.T.; Colegrave, M.; Knight, P.J.; Peckham, M. Non-muscle myosins 2A and 2B drive changes in cell morphology that occur as myoblasts align and fuse. J. Cell Sci. 2006, 119, 3561–3570. [Google Scholar] [CrossRef] [PubMed]
- Shewan, A.M.; Maddugoda, M.; Kraemer, A.; Stehbens, S.J.; Verma, S.; Kovacs, E.M. Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell-cell contacts. Mol. Biol. Cell 2005, 16, 4531–4542. [Google Scholar] [CrossRef] [PubMed]
- Sebastian, S.; Goulding, L.; Kuchipudi, S.V.; Chang, K. Extended 2D myotube culture recapitulates postnatal fibre type plasticity. BMC Cell Biol. 2015, 16, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.B.; Crow, M.T.; Stockdale, F.E. Slow and fast myosin heavy chain content defines three types of myotubes in early muscle cell cultures. J. Cell Biol. 1985, 101, 1643–1650. [Google Scholar] [CrossRef] [PubMed]
- Langhammer, G.C.; Kutzing, K.M.; Luo, V.; Zahn, D.J.; Firestein, L.B. A Topographically modified substrate-embedded MEA for directed myotube formation at electrode contact sites. Ann. Biomed. Eng. 2013, 41, 408–420. [Google Scholar] [CrossRef] [PubMed]
- Clark, P.; Dunn, G.A.; Knibbs, A.; Peckham, M. Alignment of myoblasts on ultrafine gratings inhibits fusion in vitro. Int. J. Biochem. Cell Biol. 2002, 34, 816–825. [Google Scholar] [CrossRef]
- Jensen, T.E.; Sylow, L.; Rose, A.J.; Madsen, A.B.; Angin, Y.; Maarbjerg, S.J.; Richter, E.A. Contraction-stimulated glucose transport in muscle is controlled by AMPK and mechanical stress but not sarcoplasmatic reticulum Ca2+ release. Mol. Metab. 2014, 3, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Mu, J.; Brozinick, J.T.; Valladares, O.; Bucan, M.; Birnbaum, M.J. A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 2001, 7, 1085–1094. [Google Scholar] [CrossRef]
- Merry, T.L.; McConell, G.K. Skeletal muscle glucose uptake during exercise: A focus on reactive oxygen species and nitric oxide signaling. IUBMB Life 2009, 61, 479–484. [Google Scholar] [CrossRef] [PubMed]
- Ihlemann, J.; Ploug, T.; Hellsten, Y.; Galbo, H. Effect of tension on contraction-induced glucose transport in rat skeletal muscle. Am. J. Physiol. 1999, 277, E208–E214. [Google Scholar] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCt method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]






| Target Gene | Forward Sequencing Primer (5’ to 3’) | Reverse Sequencing Primer (5’ to 3’) | Product Length | Accession Number |
|---|---|---|---|---|
| GLUT4 | GTAACTTCATTGTCGGCATGG | AGCTGAGATCTGGTCAAACG | 155 | NM_009204 |
| MyHC-1 | GTCCAAGTTCCGCAAGGT | CCACCTAAAGGGCTGTTG | 205 | NM_080728 |
| MyHC-2a | TGACCTTGAGCTGACACTGG | CGGTGCCACAGGCAAACTG | 194 | NM_001039545.2 |
| MyHC-2b | CGGTGCCACAGGCAAACTG | AGAAGCATCTCAATAAGCTCTGGTT | 150 | NM_010855.3 |
| GAPDH | CCGTGTTCCTACCCCCAATG | AAGCCCAGCTCTCCCCATA | 82 | NM_008084.3 |
| β-actin | GATTACTGCTCTGGCTCCTAG | GACTCATCGTACTCCTGCTTG | 147 | NM_007393.5 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Poosala, P.; Ichinose, H.; Kitaoka, T. Spatial Geometries of Self-Assembled Chitohexaose Monolayers Regulate Myoblast Fusion. Int. J. Mol. Sci. 2016, 17, 686. https://doi.org/10.3390/ijms17050686
Poosala P, Ichinose H, Kitaoka T. Spatial Geometries of Self-Assembled Chitohexaose Monolayers Regulate Myoblast Fusion. International Journal of Molecular Sciences. 2016; 17(5):686. https://doi.org/10.3390/ijms17050686
Chicago/Turabian StylePoosala, Pornthida, Hirofumi Ichinose, and Takuya Kitaoka. 2016. "Spatial Geometries of Self-Assembled Chitohexaose Monolayers Regulate Myoblast Fusion" International Journal of Molecular Sciences 17, no. 5: 686. https://doi.org/10.3390/ijms17050686
APA StylePoosala, P., Ichinose, H., & Kitaoka, T. (2016). Spatial Geometries of Self-Assembled Chitohexaose Monolayers Regulate Myoblast Fusion. International Journal of Molecular Sciences, 17(5), 686. https://doi.org/10.3390/ijms17050686








