Conditional Knockout in Mice Reveals the Critical Roles of Ppp2ca in Epidermis Development
Abstract
:1. Introduction
2. Results
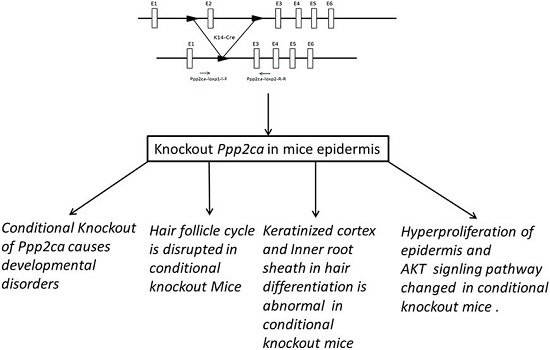
2.1. Genotyping and Tissue-Specific Recombination in Ppp2caflox/flox; Krt14-Cre Mice
2.2. Conditional Knockout of Ppp2ca Causes Developmental Disorders
2.3. Hair Follicle Cycle Is Disrupted in Ppp2caflox/flox; Krt14-Cre Mice
2.4. Ppp2ca Is Required for the Keratinized Cortex and Inner Root Sheath (IRS) in Hair Differentiation
2.5. Hyperproliferation of Epidermis in Ppp2caflox/flox; Krt14-Cre Mice and AKT Signling Pathway Changed
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Generation of Ppp2caflox/flox; Krt14-Cre Mice
- Ppp2ca-loxp1-L-F: 5′-AATAATGCGGCCGCAACCCCAACAACAACCACA-3′
- Ppp2ca-loxp2-R-R: 5′-AATAATGTCGACACCATCTACTCTAAACTCTCCACTT-3′
- Cre-F: 5′-TTGCCTGCATTACCGGTCGATGC-3′
- Cre-R: 5′-TTGCACGTTCACCGGCATCAACG-3′
4.3. RNA Preparation and Real-Time PCR
- Mpp2ca-P5F: 5′-GGTCAAGAGCCTCTGCGAGAA-3′
- Mpp2ca-P5R1: 5′-CCGGTCATGGCACCAGTTAT-3′
- Ppp2ca-qf: 5′-ATGGACGAGAAGTTGTTCACC-3′
- Ppp2ca-qr: 5′-CAGTGACTGGACATCGAACCT-3′
- Ppp2cb-qf: 5′-GAGGGTACTACTCTGTGGAGAC-3′
- Ppp2cb-qr: 5′-CCGGCTTTCGTGATTTCCT-3′
- M-β-actin-S: 5′-GTGACGTTGACATCCGTAAAGA-3′
- M-β-actin-A: 5′-GTAACAGTCCGCCTAGAAGCAC-3′
4.4. Western Blotting
4.5. Histological Analysis
4.6. Immunohistochemistry
4.7. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Liu, S.; Zhang, H.; Duan, E. Epidermal development in mammals: Key regulators, signals from beneath, and stem cells. Int. J. Mol. Sci. 2013, 14, 10869–10895. [Google Scholar] [CrossRef] [PubMed]
- Sotiropoulou, P.A.; Blanpain, C. Development and homeostasis of the skin epidermis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008383. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.M.; Chen, Y.J.; Shen, L.F.; Haddad, A.N.; Song, I.W.; Chen, L.Y.; Chen, Y.J.; Wu, J.Y.; Yen, J.J.; Chen, Y.T. Cyclic Alopecia and Abnormal Epidermal Cornification in Zdhhc13-Deficient Mice Reveal the Importance of Palmitoylation in Hair and Skin Differentiation. J. Investig. Dermatol. 2015, 135, 2603–2610. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez-Alonso, P.; Cristobal, I.; Manso, R.; Madoz-Gurpide, J.; Garcia-Foncillas, J.; Rojo, F. PP2A inhibition as a novel therapeutic target in castration-resistant prostate cancer. Tumour Biol. 2015, 36, 5753–5755. [Google Scholar] [CrossRef] [PubMed]
- Brestovitsky, A.; Sharf, R.; Mittelman, K.; Kleinberger, T. The adenovirus E4orf4 protein targets PP2A to the ACF chromatin-remodeling factor and induces cell death through regulation of SNF2h-containing complexes. Nucleic Acids Res. 2011, 39, 6414–6427. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.C.; Kuai, X.X.; Gao, W.B.; Yu, J.C.; Wang, Q.; Li, L.; Zhang, L. Morroniside-induced PP2A activation antagonizes tau hyperphosphorylation in a cellular model of neurodegeneration. J. Alzheimer’s Dis. 2016, 51, 33–44. [Google Scholar] [CrossRef] [PubMed]
- Lambrecht, C.; Haesen, D.; Sents, W.; Ivanova, E.; Janssens, V. Structure, regulation, and pharmacological modulation of PP2A phosphatases. Methods Mol. Biol. 2013, 1053, 283–305. [Google Scholar] [PubMed]
- Gotz, J.; Probst, A.; Ehler, E.; Hemmings, B.; Kues, W. Delayed embryonic lethality in mice lacking protein phosphatase 2A catalytic subunit Cα. Proc. Natl. Acad. Sci. USA 1998, 95, 12370–12375. [Google Scholar] [CrossRef] [PubMed]
- Ciccone, M.; Calin, G.A.; Perrotti, D. From the biology of PP2A to the PADs for therapy of hematologic malignancies. Front. Oncol. 2015, 5, 21. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Chen, X.; Tong, X.; Tang, C.; Li, J. Ppp2ca knockout in mice spermatogenesis. Reproduction 2015, 149, 385–391. [Google Scholar] [CrossRef] [PubMed]
- Hafner, M.; Wenk, J.; Nenci, A.; Pasparakis, M.; Scharffetter-Kochanek, K.; Smyth, N.; Peters, T.; Kess, D.; Holtkotter, O.; Shephard, P.; et al. Keratin 14 Cre transgenic mice authenticate keratin 14 as an oocyte-expressed protein. Genesis 2004, 38, 176–181. [Google Scholar] [CrossRef] [PubMed]
- Courtois, M.; Loussouarn, G.; Hourseau, C.; Grollier, J.F. Hair cycle and alopecia. Skin Pharmacol. 1994, 7, 84–89. [Google Scholar] [CrossRef] [PubMed]
- Qiu, W.; Li, X.; Tang, H.; Huang, A.S.; Panteleyev, A.A.; Owens, D.M.; Su, G.H. Conditional activin receptor type 1B (Acvr1b) knockout mice reveal hair loss abnormality. J. Investig. Dermatol. 2011, 131, 1067–1076. [Google Scholar] [CrossRef] [PubMed]
- Lynch, M.H.; O’Guin, W.M.; Hardy, C.; Mak, L.; Sun, T.T. Acidic and basic hair/nail (“hard”) keratins: their colocalization in upper cortical and cuticle cells of the human hair follicle and their relationship to “soft” keratins. J. Cell Biol. 1986, 103, 2593–2606. [Google Scholar] [CrossRef] [PubMed]
- Moravcova, M.; Libra, A.; Dvorakova, J.; Viskova, A.; Muthny, T.; Velebny, V.; Kubala, L. Modulation of keratin 1, 10 and involucrin expression as part of the complex response of the human keratinocyte cell line HaCaT to ultraviolet radiation. Interdiscip. Toxicol. 2013, 6, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Wang, Z.; Jiang, C.; Ding, Y. PP2A-mediated anticancer therapy. Gastroenterol. Res. Pract. 2013, 2013, 675429. [Google Scholar] [CrossRef] [PubMed]
- Planel, E.; Richter, K.E.; Nolan, C.E.; Finley, J.E.; Liu, L.; Wen, Y.; Krishnamurthy, P.; Herman, M.; Wang, L.; Schachter, J.B.; et al. Anesthesia leads to tau hyperphosphorylation through inhibition of phosphatase activity by hypothermia. J. Neurosci. 2007, 27, 3090–3097. [Google Scholar] [CrossRef] [PubMed]
- Whittington, R.A.; Virag, L.; Marcouiller, F.; Papon, M.A.; El Khoury, N.B.; Julien, C.; Morin, F.; Emala, C.W.; Planel, E. Propofol directly increases tau phosphorylation. PLoS ONE 2011, 6, e16648. [Google Scholar] [CrossRef] [PubMed]
- Spurgeon, M.E.; Cheng, J.; Bronson, R.T.; Lambert, P.F.; DeCaprio, J.A. Tumorigenic activity of merkel cell polyomavirus T antigens expressed in the stratified epithelium of mice. Cancer Res. 2015, 75, 1068–1079. [Google Scholar] [CrossRef] [PubMed]
- Pandey, P.; Seshacharyulu, P.; Das, S.; Rachagani, S.; Ponnusamy, M.P.; Yan, Y.; Johansson, S.L.; Datta, K.; Fong Lin, M.; Batra, S.K. Impaired expression of protein phosphatase 2A subunits enhances metastatic potential of human prostate cancer cells through activation of AKT pathway. Br. J. Cancer 2013, 108, 2590–2600. [Google Scholar] [CrossRef] [PubMed]
- Ugi, S.; Imamura, T.; Maegawa, H.; Egawa, K.; Yoshizaki, T.; Shi, K.; Obata, T.; Ebina, Y.; Kashiwagi, A.; Olefsky, J.M. Protein phosphatase 2A negatively regulates insulin’s metabolic signaling pathway by inhibiting Akt (protein kinase B) activity in 3T3-L1 adipocytes. Mol. Cell. Biol. 2004, 24, 8778–8789. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Carracedo, D.; Angeles Villaronga, M.; Alvarez-Teijeiro, S.; Hermida-Prado, F.; Santamaria, I.; Allonca, E.; Suarez-Fernandez, L.; Victoria Gonzalez, M.; Balbin, M.; Astudillo, A.; et al. Impact of PI3K/AKT/mTOR pathway activation on the prognosis of patients with head and neck squamous cell carcinomas. Oncotarget 2016. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.Y.; Kang, M.B.; Jang, S.H.; Qian, T.; Kim, H.J.; Kim, C.H.; Kim, Y.; Kong, G. Id-1 activates Akt-mediated Wnt signaling and p27Kip1 phosphorylation through PTEN inhibition. Oncogene 2009, 28, 824–831. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, C.; Distler, J.H. Canonical Wnt signaling in systemic sclerosis. Lab. Investig. 2016, 96, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Rishikaysh, P.; Dev, K.; Diaz, D.; Qureshi, W.M.; Filip, S.; Mokry, J. Signaling involved in hair follicle morphogenesis and development. Int. J. Mol. Sci. 2014, 15, 1647–1670. [Google Scholar] [CrossRef] [PubMed]
- Bai, J.; Liu, Z.; Xu, Z.; Ke, F.; Zhang, L.; Zhu, H.; Lou, F.; Wang, H.; Fei, Y.; Shi, Y.L.; et al. Epigenetic downregulation of SFRP4 contributes to epidermal hyperplasia in psoriasis. J. Immunol. 2015, 194, 4185–4198. [Google Scholar] [CrossRef] [PubMed]
- Wlodarchak, N.; Xing, Y. PP2A as a master regulator of the cell cycle. Crit. Rev. Biochem. Mol. Biol. 2016, 51, 162–184. [Google Scholar] [CrossRef] [PubMed]
- Perrotti, D.; Neviani, P. Protein phosphatase 2A: A target for anticancer therapy. Lancet Oncol. 2013, 14, e229–e238. [Google Scholar] [CrossRef]
- Clevers, H. Wnt/β-catenin signaling in development and disease. Cell 2006, 127, 469–480. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.H.; Seeling, J.M.; Hill, V.; Yochum, A.; Virshup, D.M. Casein kinase I phosphorylates and destabilizes the β-catenin degradation complex. Proc. Natl. Acad. Sci. USA 2002, 99, 1182–1187. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yost, H.J.; Virshup, D.M.; Seeling, J.M. Protein phosphatase 2A and its B56 regulatory subunit inhibit Wnt signaling in Xenopus. EMBO J. 2001, 20, 4122–4131. [Google Scholar] [CrossRef] [PubMed]
- Byrne, C.; Tainsky, M.; Fuchs, E. Programming gene expression in developing epidermis. Development 1994, 120, 2369–2383. [Google Scholar] [PubMed]
- Wang, X.P.; Suomalainen, M.; Jorgez, C.J.; Matzuk, M.M.; Werner, S.; Thesleff, I. Follistatin regulates enamel patterning in mouse incisors by asymmetrically inhibiting BMP signaling and ameloblast differentiation. Dev. Cell 2004, 7, 719–730. [Google Scholar] [CrossRef] [PubMed]







© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fang, C.; Li, L.; Li, J. Conditional Knockout in Mice Reveals the Critical Roles of Ppp2ca in Epidermis Development. Int. J. Mol. Sci. 2016, 17, 756. https://doi.org/10.3390/ijms17050756
Fang C, Li L, Li J. Conditional Knockout in Mice Reveals the Critical Roles of Ppp2ca in Epidermis Development. International Journal of Molecular Sciences. 2016; 17(5):756. https://doi.org/10.3390/ijms17050756
Chicago/Turabian StyleFang, Chao, Lei Li, and Jianmin Li. 2016. "Conditional Knockout in Mice Reveals the Critical Roles of Ppp2ca in Epidermis Development" International Journal of Molecular Sciences 17, no. 5: 756. https://doi.org/10.3390/ijms17050756