Impact of Faba Bean-Seed Rhizobial Inoculation on Microbial Activity in the Rhizosphere Soil during Growing Season
Abstract
:1. Introduction
2. Results and Discussion
2.1. Enzyme Activities
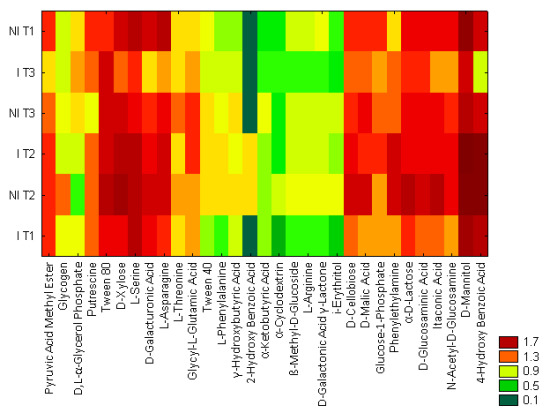
2.2. Community Level Physiological Profiling (CLPP)
3. Materials and Methods
3.1. Description of the Study Site and Treatments
3.2. Sampling of Rhizosphere Soil
3.3. Enzymatic Activities
3.4. Community Level Physiological Profiling
3.5. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Yadav, J.; Verma, J.P. Effect of seed inoculation with indigenous Rhizobium and plant growth promoting rhizobacteria on nutrients uptake and yields of chickpea (Cicer arietinum L.). Eur. J. Soil Biol. 2014, 63, 70–77. [Google Scholar] [CrossRef]
- Denton, M.D.; Pearce, D.J.; Peoples, M.B. Nitrogen contributions from faba bean (Vicia faba L.) reliant on soil rhizobia or inoculation. Plant Soil 2013, 365, 363–374. [Google Scholar] [CrossRef]
- Trabelsi, D.; Mengoni, A.; Ammar, H.B.; Mhamdi, R. Effect of on-field inoculation of Phaseolus vulgaris with rhizobia on soil bacterial communities. FEMS Microbiol. Ecol. 2011, 77, 211–222. [Google Scholar] [CrossRef] [PubMed]
- Trabelsi, D.; Ammar, H.B.; Mengoni, A.; Mhamdi, R. Appraisal of the crop-rotation effect of rhizobial inoculation on potato cropping systems in relation to soil bacterial communities. Soil Biol. Biochem. 2012, 54, 1–6. [Google Scholar] [CrossRef]
- Zahir, A.Z.; Zafar-ul-Hye, M.; Sajjad, S.; Naveed, M. Comparative effectiveness of Pseudomonas and Serratia sp. containing ACC-deaminase for coinoculation with Rhizobium leguminosarum to improve growth, nodulation, and yield of lentil. Biol. Fertil. Soils 2011, 47, 457–465. [Google Scholar] [CrossRef]
- Flores-Félix, J.D.; Menéndez, E.; Rivera, L.P.; Marcos-García, M.; Martínez-Hidalgo, P.; Mateos, P.F.; Martínez-Molina, E.; Velázquez, E.; García-Fraile, P.; Rivas, R. Use of Rhizobium leguminosarum as a potential biofertilizer for Lactuca sativa and Daucus carota crops. J. Plant Nutr. Soil Sci. 2013, 176, 876–882. [Google Scholar]
- Antoun, H.; Prévost, D. Ecology of plant growth promoting rhizobacteria. In PGPR: Biocontrol and Biofertilization; Siddiqui, Z.A., Ed.; Springer: Dordrecht, The Netherlands, 2005; pp. 1–38. [Google Scholar]
- Bardin, S.D.; Huang, H.C.; Pinto, J.; Amundsen, E.J.; Erickson, R.S. Biological control of Pythium damping-off of pea and sugar beet by Rhizobium leguminosarum bv. viceae. Can. J. Bot. 2004, 82, 291–296. [Google Scholar] [CrossRef]
- Huang, H.C.; Erickson, R.S. Effect of seed treatment with Rhizobium leguminosarum bv. viceae on Pythium damping-off, seedling height, root nodulation, root biomass, shoot biomass, and seed yield of pea and lentil. J. Phytopathol. 2007, 155, 31–37. [Google Scholar] [CrossRef]
- Shaban, W.I.; El-Bramawy, M.A. Impact of dual inoculation with Rhizobium and Trichoderma on damping off, root rot diseases and plant growth parameters of some legumes field crop under greenhouse conditions. Int. Res. J. Agric. Sci. Soil Sci. 2011, 1, 98–108. [Google Scholar]
- Lipiec, J.; Gliński, J. Rhizosphere. In Encyclopedia of Agrophysics; Gliński, J., Horabik, J., Lipiec, J., Eds.; Springer: Dordrecht, The Netherlands, 2011; pp. 705–709. [Google Scholar]
- Parmar, N.; Dadarwal, K.R. Rhizobacteria from rhizosphere and rhizoplane of chick pea (Cicer arietinum L.). Indian J. Microbiol. 1997, 37, 205–210. [Google Scholar]
- Florek, J.; Czerwińska-Kayzer, D.; Jerzak, M.A. Aktualny stan i wykorzystanie produkcji upraw roślin strączkowych. Fragm. Agron. 2012, 29, 45–55. [Google Scholar]
- Alef, K.; Nannipieri, P.; Trazar-Cepeda, C. Phosphatase activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 335–344. [Google Scholar]
- Paul, E.A.; Clark, F.E. Soil Biology and Biochemistry, 2nd ed.; Academic Press: San Diego, CA, USA, 1996; 188p. [Google Scholar]
- Schimel, J.P.; Bennett, J. Nitrogen mineralization: Challenges of a changing paradigm. Ecology 2004, 85, 591–602. [Google Scholar] [CrossRef]
- Gopalakrishnan, S.; Sathya, A.; Vijayabharathi, R.; Varshney, R.K.; Laxmipathi Gowda, C.L.; Krishnamurthy, L. Plant growth promoting rhizobia: Challenges and opportunities. 3 Biotech 2015, 5, 355–377. [Google Scholar] [CrossRef] [Green Version]
- Liu, B.; Li, Y.; Zhang, X.; Wang, J.; Gao, M. Effects of chlortetracycline on soil microbial communities: Comparisons of enzyme activities to the functional diversity via Biolog EcoPlates™. Eur. J. Soil Biol. 2015, 68, 69–76. [Google Scholar] [CrossRef]
- World Reference Base for Soil Resources 2014: International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; Food and Agriculture Organization of the United Nations: Rome, Italy, 2015; Available online: http://www.fao.org/3/a-i3794e.pdf (accessed on 4 April 2016).
- Thalmann, A. Zur methodik der bestimmung der dehydrogenase activität im boden mittels triphenyltetrazoliumchlorid (TTC). Landwirtsh. Forsch. 1968, 21, 249–258. [Google Scholar]
- Alef, K. Dehydrogenase activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: London, UK, 1995; pp. 228–231. [Google Scholar]
- Zantua, M.I.; Bremner, J.M. Stability of urease in soils. Soil Biol. Biochem. 1977, 9, 135–140. [Google Scholar] [CrossRef]
- Ladd, J.N.; Butler, J.H. Short-term assays of soil proteolytic enzyme activities using proteins and dipeptide derivatives as substrates. Soil Biol. Biochem. 1972, 4, 19–30. [Google Scholar] [CrossRef]
- Alef, K.; Nannipieri, P. Protease activity. In Methods in Applied Soil Microbiology and Biochemistry; Alef, K., Nannipieri, P., Eds.; Academic Press: San Diego, CA, USA, 1995; pp. 313–315. [Google Scholar]
- Tabatabai, M.; Bremner, J. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Insam, H. A new set of substrates proposed for community characterization in environmental samples. In Microbial Communities. Functional versus Structural Approach; Insam, H., Rangger, A., Eds.; Springer: Berlin, Germany; Heidelberg, Germany, 1997; pp. 259–260. [Google Scholar]
- Garland, J.L.; Millis, A.L. Classification and characterization of heterotrophic microbial communities on the basis of patterns of community-level sole-carbon-source utilization. Appl. Environ. Microbiol. 1999, 57, 2351–2359. [Google Scholar]
- Garland, J.L. Analysis and interpretation of community-level physiological profiles in microbial ecology. FEMS Microbiol. Ecol. 1997, 24, 289–300. [Google Scholar] [CrossRef]




| Time | Treatment | CLPP | ||
|---|---|---|---|---|
| AWCD | R | H | ||
| T1 | NI | 1.13 a | 30.0 ab | 3.376 b |
| I | 1.15 a | 30.0 ab | 3.391 b | |
| T2 | NI | 1.32 a | 30.7 ab | 3.394 ab |
| I | 1.25 a | 31.0 a | 3.392 b | |
| T3 | NI | 1.17 a | 30.3 ab | 3.398 ab |
| I | 1.08 a | 29.7 b | 3.419 a | |
| Means | NI | 1.21 a | 30.2 a | 3.389 b |
| I | 1.16 a | 30.3 a | 3.401 a | |
| Factors | Enzyme Activities | CLPP | |||||
|---|---|---|---|---|---|---|---|
| Dehydrogenases (µg TPF g−1·d−1) | Urease (mg N-NH4 kg−1·h−1) | Protease (mg Tyrosine kg−1·h−1) | Acid Phosphomonoesterase (mmol PNP kg−1·h−1) | AWCD | R | H | |
| Inoculation | 496.378 *** | 406.903 *** | 3.3857 | 34.9 *** | 1.233 | 0.33 | 7.09 * |
| Time | 316.912 *** | 57.686 *** | 8.8197 ** | 60.65 *** | 6.047 * | 8.33 ** | 10.39 ** |
| Inoculation*Time | 35.981 *** | 15.03 *** | 0.7304 | 4.05 * | 0.521 | 2.33 | 2.46 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siczek, A.; Lipiec, J. Impact of Faba Bean-Seed Rhizobial Inoculation on Microbial Activity in the Rhizosphere Soil during Growing Season. Int. J. Mol. Sci. 2016, 17, 784. https://doi.org/10.3390/ijms17050784
Siczek A, Lipiec J. Impact of Faba Bean-Seed Rhizobial Inoculation on Microbial Activity in the Rhizosphere Soil during Growing Season. International Journal of Molecular Sciences. 2016; 17(5):784. https://doi.org/10.3390/ijms17050784
Chicago/Turabian StyleSiczek, Anna, and Jerzy Lipiec. 2016. "Impact of Faba Bean-Seed Rhizobial Inoculation on Microbial Activity in the Rhizosphere Soil during Growing Season" International Journal of Molecular Sciences 17, no. 5: 784. https://doi.org/10.3390/ijms17050784
APA StyleSiczek, A., & Lipiec, J. (2016). Impact of Faba Bean-Seed Rhizobial Inoculation on Microbial Activity in the Rhizosphere Soil during Growing Season. International Journal of Molecular Sciences, 17(5), 784. https://doi.org/10.3390/ijms17050784