Extraction Optimization, Preliminary Characterization and Bioactivities in Vitro of Ligularia hodgsonii Polysaccharides
Abstract
:1. Introduction
2. Results and Discussion
2.1. Optimization Extraction of the Ligularia hodgsonii Hook. (LH) Polysaccharides
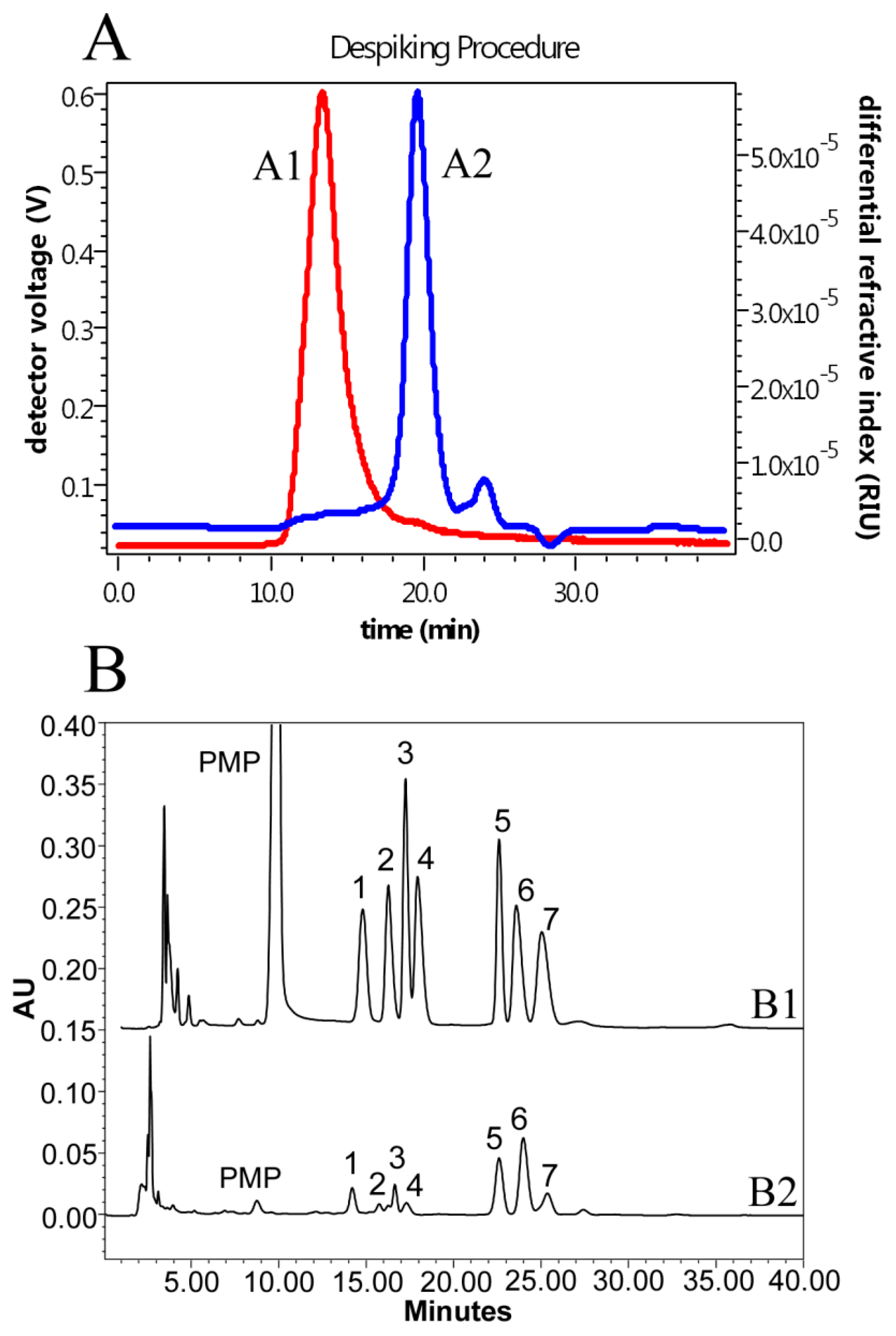
2.2. Preliminary Characterization of the LH Polysaccharides
2.3. Antioxidant Activities
2.3.1. 1,1-Diphenyl-2-picrylhydrazyl (DPPH) Radical Scavenging Activity
2.3.2. 2,2′-Azinobis (3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) Radical Scavenging Activity
2.3.3. Hydroxyl Radical Scavenging Activity
2.3.4. Ferrous Metal Ions Chelating Activity
2.3.5. Reducing Power
2.4. In Vitro Anti-Hyperglycemic Study
2.4.1. α-Glucosidase Inhibitory Activity
2.4.2. α-Amylase Inhibitory Activity
3. Materials and Methods
3.1. Materials and Reagents
3.2. Extraction of Crude Polysaccharides
3.3. Single Factor Experiments
3.4. Orthogonal Array Test Design
3.5. Removal of Impurities in Crude Polysaccharides
3.6. Preliminary Characterization of the Polysaccharides
3.6.1. General Methods
3.6.2. Analysis of Monosaccharide Composition
3.6.3. Determination of Molecular Weight
3.6.4. Infrared (IR) Analysis
3.6.5. 1H Nuclear Magnetic Resonance (1H NMR)
3.7. In Vitro Antioxidant Activity Assays
3.7.1. DPPH Radical Scavenging Assay
3.7.2. ABTS Radical Scavenging Assay
3.7.3. Hydroxyl Radical Scavenging Assay
3.7.4. Ferrous Metal Ions Chelating Activity
3.7.5. Ferric Reducing Power (FRP)
3.8. In Vitro Anti-Hyperglycemic Studies
3.8.1. α-Glucosidase Inhibitory Activity
3.8.2. α-Amylase Inhibitory Activity
3.9. Statistical Analyses
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Zhou, Z.; Tang, J.; Song, X. Chemical composition of the essential oil of Ligularia hodgsonii and free radical scavenging activity of the oil and crude extracts. Nat. Prod. Commun. 2014, 9, 1511–1514. [Google Scholar] [PubMed]
- Liu, C.L.; Li, Y.; Du, N.; Huang, X.; Wang, W.S. Study on the purification and scavenging free radical activity of water soluble polysaccharide from Ligularia hodgsonii. Chin. Med. Mater. 2010, 33, 1414–1416. [Google Scholar]
- Liu, C.L.; Du, N.; Xu, G.Y.; Huang, X.; A, X.N. Isolation, purification and structural analysis of water-soluble polysaccharide LW21 from Ligularia hodgsonii. Food Sci. 2012, 33, 57–61. [Google Scholar]
- Yang, H.; Jin, X.; Lam, K.; Wai, C.; Yan, S.K. Oxidative stress and diabetes mellitus. Clin. Chem. Lab. Med. 2011, 49, 1773–1782. [Google Scholar] [CrossRef] [PubMed]
- Filomeni, G.; de Zio, D.; Cecconi, F. Oxidative stress and autophagy: The clash between damage and metabolic needs. Cell Death Differ. 2015, 22, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Ernst, B.; Magnani, J.L. From carbohydrate leads to glycomimetic drugs. Nat. Rev. Drug Discov. 2009, 8, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.T.; Rioux, L.E.; Turgeon, S.L. α-Amylase and α-glucosidase inhibition is differentially modulated by fucoidan obtained from Fucus vesiculosus and Ascophyllum nodosum. Phytochemistry 2014, 98, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Chen, J.; Li, E.T.; Fan, Q.; Wang, D.Y.; Li, P.; Li, X.P.; Chen, X.Y.; Qiu, S.L.; Gao, Z.Z.; et al. The comparison of antioxidative and hepatoprotective activities of Codonopsis pilosula polysaccharide (CP) and sulfated CP. Int. Immunopharmacol. 2015, 24, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Son, Y.O.; Kim, S.S.; Jang, Y.S.; Lee, J.C. Antioxidant and anti-hyperglycemic activity of polysaccharide isolated from Dendrobium chrysotoxum Lindl. J. Biochem. Mol. Biol. 2007, 40, 670–677. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Cheng, M.; Hattori, M. Pyrrolizidine alkaloid profile in a traditional Chinese herbal medicine Chuan Zi Wan (Ligulariae Radix et Rhizoma) by liquid chromatography /electrospray ionization ion trap mass spectrometry. Anal. Methods 2012, 4, 2797–2808. [Google Scholar] [CrossRef]
- Ji, L.L.; Liu, T.Y.; Wang, Z.T. Pyrrolizidine alkaloid clivorine induced oxidative injury on primary cultured rat hepatocytes. Hum. Exp. Toxicol. 2010, 29, 303–309. [Google Scholar] [PubMed]
- Cheng, M.; Tang, J.; Gao, Q.F.; Lin, G. Analysis on clivorine from alkaloid in aqueous extract of Ligularia hodgsonii and its hepatotoxicity in rats. Chin. Tradit. Herb. Drugs 2011, 42, 2507–2511. [Google Scholar]
- Yang, W.; Wang, Y.; Li, X.; Yu, P. Purification and structural characterization of Chinese yam polysaccharide and its activities. Carbohydr. Polym. 2015, 117, 1021–1027. [Google Scholar] [CrossRef] [PubMed]
- Zeng, H.; Zhang, Y.; Zhao, Y.; Tian, Y.; Miao, S.; Zheng, B. Extraction optimization, structure and antioxidant activities of Fortunella margarita Swingle polysaccharides. Int. J. Biol. Macromol. 2015, 74, 232–242. [Google Scholar] [CrossRef] [PubMed]
- Ismail, B.; Nampoothiri, K.M. Production, purification and structural characterization of an exopolysaccharide produced by a probiotic Lactobacillus plantarum MTCC 9510. Arch. Microbiol. 2010, 192, 1049–1057. [Google Scholar] [CrossRef] [PubMed]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized methods for the determination of antioxidant capacity and phenolics in foods and dietary supplements. J. Agric. Food Chem. 2005, 53, 4290–4302. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.L.; Zheng, S.L.; Fan, Q.J.; Yuan, J.C.; Yang, S.M.; Kong, F.L. Optimisation of high-pressure ultrasonic-assisted extraction and antioxidant capacity of polysaccharides from the rhizome of Ligusticum chuanxiong. Int. J. Biol. Macromol. 2015, 76, 80–85. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.Q.; Chen, J.C.; Du, H.T.; Li, Q.; Chen, J.; Zhang, G.C.; Liu, H.; Wang, J.R. Structural characterization and antioxidant activities of polysaccharides extracted from the pulp of Elaeagnus angustifolia L. Int. J. Mol. Sci. 2014, 15, 11446–11455. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Morris, H.; Cronin, M.T.D. Metals, toxicity and oxidative stress. Curr. Med. Chem. 2005, 12, 1161–1208. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.L.; Wang, C.C.; Chang, S.C.; Inbaraj, B.S.; Chen, B.H. Antioxidative activity of polysaccharide fractions isolated from Lycium barbarum Linnaeus. Int. J. Biol. Macromol. 2009, 45, 146–151. [Google Scholar] [CrossRef] [PubMed]
- Fan, J.; Wu, Z.; Zhao, T.; Sun, Y.; Ye, H.; Xu, R.; Zeng, X. Characterization, antioxidant and hepatoprotective activities of polysaccharides from Ilex latifolia Thunb. Carbohydr. Polym. 2014, 101, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.H.; Yu, Y.; Liang, Y.Z.; Chen, X.Q. Purification, partial characterization and antioxidant activity of polysaccharides from Glycyrrhiza uralensis. Int. J. Biol. Macromol. 2015, 79, 681–686. [Google Scholar] [CrossRef] [PubMed]
- De Melo, E.B.; da Silveira Gomes, A.; Carvalho, I. α-and β-Glucosidase inhibitors: chemical structure and biological activity. Tetrahedron 2006, 62, 10277–10302. [Google Scholar] [CrossRef]
- Bisht, S.; Kant, R.; Kumar, V. α-d-Glucosidase inhibitory activity of polysaccharide isolated from Acacia tortilis gum exudate. Int. J. Biol. Macromol. 2013, 59, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, Z.; Wei, X. Sugar compositions, α-glucosidase inhibitory and amylase inhibitory activities of polysaccharides from leaves and flowers of Camellia sinensis obtained by different extraction methods. Int. J. Biol. Macromol. 2010, 47, 534–539. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.Q.; Li, M.; Zhu, F.; Liu, F.L.; Huang, J.B. Inhibitory potential of trilobatin from Lithocarpus polystachyus Rehd against α-glucosidase and α-amylase linked to type 2 diabetes. Food Chem. 2012, 130, 261–266. [Google Scholar] [CrossRef]
- Sun, Y.; Wang, H.; Guo, G.; Pu, Y.; Yan, B. The isolation and antioxidant activity of polysaccharides from the marine microalgae Isochrysis galbana. Carbohydr. Polym. 2014, 113, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Dubois, M.; Gilles, K.A.; Hamilton, J.K.; Rebers, P.A.; Smith, F. Calorimetric method for determination of sugars and related substances. Anal. Chem. 1956, 28, 350–356. [Google Scholar] [CrossRef]
- Zhao, Y.Z.; Lu, C.T.; Mei, X.G. Multi-Index test applied in orthogonal design. J. Med. Postgrad. 2004, 17, 624–626. [Google Scholar]
- Staub, A.M. Removal of protein-Sevag method. Methods Carbohydr. Chem. 1965, 5, 5–6. [Google Scholar]
- Bradford, M.M. A rapid method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar]
- Blumenkrantz, N.; Asboe-Hansen, G. New method for quantitative determination of uronic acid. Anal. Biochem. 1973, 54, 484–489. [Google Scholar] [CrossRef]
- Dodgson, K.S.; Price, R.G. A note on the determination of the ester sulphate content of sulphated polysaccharides. Biochem. J. 1962, 84, 106–110. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Yang, X.; Zhao, Y.; Ruan, Y.; Yang, Y.; Wang, Z. Separation and quantification of component monosaccharides of the tea polysaccharides from Gynostemma pentaphyllum by HPLC with indirect UV detection. Food Chem. 2009, 112, 742–746. [Google Scholar] [CrossRef]
- Xu, S.; Xu, X.; Zhang, L. Effect of heating on chain conformation of branched β-glucan in water. J. Phys. Chem. B 2013, 117, 8370–8377. [Google Scholar] [CrossRef] [PubMed]





| No. | A (°C) | B (h) | C (mL/g) | D (Times) | Polysaccharide Yield/% | Proteins/% | Total Polyphenols/% | Clivorine/(×10−4) % | Z-Comprehensive Scoring |
|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 (80) | 1 (2) | 1 (24) | 1 (1) | 21.5 ± 1.1 | 2.15 ± 0.06 | 1.73 ± 0.04 | 2.29 ± 0.04 | 2.78 ± 0.57 |
| 2 | 1 | 2 (3) | 2 (30) | 2 (2) | 27.7 ± 0.7 | 1.94 ± 0.01 | 1.73 ± 0.03 | 1.87 ± 0.03 | 5.29 ± 0.23 |
| 3 | 1 | 3 (4) | 3 (36) | 3 (3) | 26.9 ± 1.9 | 2.50 ± 0.11 | 1.86 ± 0.02 | 1.99 ± 0.02 | 3.93 ± 0.56 |
| 4 | 2 (85) | 1 | 2 | 3 | 32.1 ± 2.2 | 2.16 ± 0.09 | 1.87 ± 0.03 | 2.11 ± 0.04 | 5.57 ± 0.45 |
| 5 | 2 | 2 | 3 | 1 | 27.6 ± 2.4 | 2.22 ± 0.10 | 1.69 ± 0.02 | 0.83 ± 0.01 | 6.35 ± 0.76 |
| 6 | 2 | 3 | 1 | 2 | 29.1 ± 1.4 | 2.22 ± 0.06 | 1.79 ± 0.03 | 1.27 ± 0.01 | 5.87 ± 0.47 |
| 7 | 3 (90) | 1 | 3 | 2 | 28.7 ± 1.0 | 2.01 ± 0.06 | 1.81 ± 0.01 | 1.40 ± 0.01 | 5.78 ± 0.29 |
| 8 | 3 | 2 | 1 | 3 | 28.1 ± 1.3 | 2.46 ± 0.06 | 1.87 ± 0.04 | 1.80 ± 0.02 | 4.58 ± 0.39 |
| 9 | 3 | 3 | 2 | 1 | 22.7 ± 2.7 | 1.91 ± 0.07 | 1.77 ± 0.03 | 0.91 ± 0.01 | 4.87 ± 0.61 |
| k1 a | 3.99 | 4.74 | 4.40 | 4.57 | - | - | - | - | - |
| k2 a | 5.98 | 5.37 | 5.25 | 5.68 | - | - | - | - | - |
| k3 a | 5.04 | 4.89 | 5.35 | 4.75 | - | - | - | - | - |
| R b | 1.99 | 0.62 | 0.96 | 1.12 | - | - | - | - | - |
| Indices | DLHP | LHP | Water Extract |
|---|---|---|---|
| Total carbohydrates (%) | 86.9 ± 0.4 | 65.2 ± 0.5 | 12.0 ± 0.5 |
| Protein (%) | 1.04 ± 0.02 | 2.25 ± 0.04 | 1.53 ± 0.03 |
| Total polyphenols (%) | 0.513 ± 0.008 | 1.68 ± 0.01 | 22.7 ± 0.3 |
| Clivorine (%) | ND c | 0.819 ± 0.009 (×10−4) | 0.394 ± 0.008 e |
| Uronic acids (%) | 5.03 ± 0.08 | 4.05 ± 0.08 | - |
| Sulfuric radical (%) | ND c | ND c | - |
| Molecular weight (×105 Da) | 1.17 ± 0.03 | 0.11 ± 0.01 | - |
| MW/Mn d | 1.42 (±4.51%) | 1.15 (±11.8%) | - |
| Mass Fraction/% | 91.6 ± 0.8 | 99.3 ± 0.4 | - |
| Samples | DPPH• Scavenging | ABTS•+ Scavenging | •OH Scavenging | Fe2+ Chelating | Inhibition of α-Glucosidase | Inhibition of α-Amylase |
|---|---|---|---|---|---|---|
| DLHP | 142.3 ± 2.0 | 62.4 ± 0.3 | 621.7 ± 6.7 | 1511.3 ± 7.6 | 358.4 ± 0.6 | (21.2 ± 0.3) × 103 |
| LHP | 127.1 ± 2.4 | 61.4 ± 0.7 | 1530.7 ± 14.3 | 5181.3 ± 78.1 | 2491.7 ± 132.8 | (114.8 ± 3.6) × 103 f |
| Water extract | 62.3 ± 0.8 | 48.5 ± 0.3 | 1346.0 ± 13.2 | 8735.3 ± 325.7 | 1604.1 ± 3.9 | (38.4 ± 3.4) × 103 f |
| Clivorine | / | / | 435.7 ± 3.8 | / | / | (6.67 ± 0.03) × 103 |
| VC | 4.71 ± 0.02 | 1.66 ± 0.01 | 87.3 ± 0.1 | / | - | - |
| BHT | 25.1 ± 0.1 | - | / | - | - | - |
| Trolox | 4.15 ± 0.02 | 4.94 ± 0.05 | 183.3 ± 1.6 | / | - | - |
| EDTA | - | - | - | 28.7 ± 0.2 | - | - |
| Acarbose | - | - | - | - | 242.0 ± 3.7 | 15.5 ± 0.3 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, X.; Tang, J. Extraction Optimization, Preliminary Characterization and Bioactivities in Vitro of Ligularia hodgsonii Polysaccharides. Int. J. Mol. Sci. 2016, 17, 788. https://doi.org/10.3390/ijms17050788
Song X, Tang J. Extraction Optimization, Preliminary Characterization and Bioactivities in Vitro of Ligularia hodgsonii Polysaccharides. International Journal of Molecular Sciences. 2016; 17(5):788. https://doi.org/10.3390/ijms17050788
Chicago/Turabian StyleSong, Xueping, and Jun Tang. 2016. "Extraction Optimization, Preliminary Characterization and Bioactivities in Vitro of Ligularia hodgsonii Polysaccharides" International Journal of Molecular Sciences 17, no. 5: 788. https://doi.org/10.3390/ijms17050788