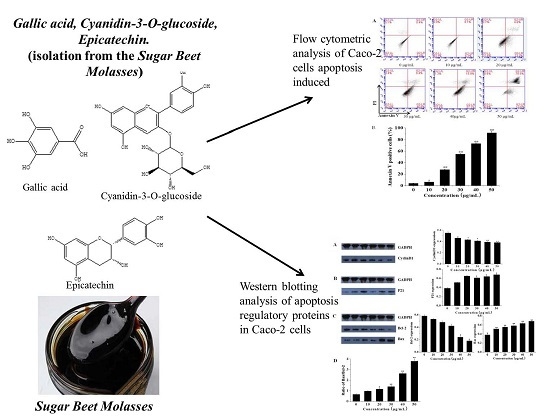
Cytotoxicity and Apoptotic Effects of Polyphenols from Sugar Beet Molasses on Colon Carcinoma Cells in Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Extraction and Isolation of SBM
2.3. Cell Lines and Cell Culture
2.4. Cytotoxicity Assay
2.5. Cell Cycle Analysis by Flow Cytometry
2.6. Cell Apoptosis Analysis by Flow Cytometry
2.7. Caspase-3 Activity Assay
2.8. Western Blot Assay
2.9. Statistical Analysis
3. Results and Discussion
3.1. Evaluation of Cytotoxicity against Tumor Cells
3.2. Changes of Cell Cycle Detected by Flow Cytometric Analysis
3.3. Flow Cytometric Analysis of Cell Apoptosis
3.4. CGC Activated Caspase-3
3.5. Effects of CGC on the mRNA and Protein Expression of Cell Cycle Protein (Cyclin D1)
3.6. CGC Upregulated the Ratio of Bax/Bcl-2 and Downregulated the Expression of Mutant p21
4. Discussion
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Wang, S.; Penchala, S.; Prabhu, S.; Wang, J.; Huang, Y. Molecular basis of traditional Chinese medicine in cancer chemoprevention. Curr. Drug Discov. Technol. 2010, 7, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Jiang, Y.; Wu, K.; Wang, S.; Wang, Y. Evaluation of antitumor property of extracts and steroidal alkaloids from the cultivated Bulbus Fritillariae ussuriensis and preliminary investigation of its mechanism of action. BMC Complement. Altern. Med. 2015, 15, 29. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Zhu, F.; Yang, Y.; Li, M. Purification, antitumor activity in vitro of steroidal glycoalkaloids from black nightshade (Solanum nigrum L.). Food Chem. 2013, 141, 1181–1186. [Google Scholar] [CrossRef] [PubMed]
- Efferth, T.; Li, P.C.; Konkimalla, V.S.B.; Kaina, B. From traditional Chinese medicine to rational cancer therapy. Trends Mol. Med. 2007, 13, 353–361. [Google Scholar] [CrossRef] [PubMed]
- Liang, J.; Li, F.; Fang, Y.; Yang, W.; An, X.; Zhao, L.; Xin, Z.; Cao, L.; Hu, Q. Cytotoxicity and apoptotic effects of tea polyphenol-loaded chitosan nanoparticles on human hepatoma HepG2 cells. Mater. Sci. Eng. C 2014, 36, 7–13. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Meng, H.; Zhao, Y.; Chen, F.; Yu, S. Antioxidant and in vitro anticancer activities of phenolics isolated from sugar beet molasses. BMC Complement. Altern. Med. 2015, 15, 313. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhao, Y.; Yu, S. Optimisation of ultrasonic-assisted extraction of phenolic compounds, antioxidants, and anthocyanins from sugar beet molasses. Food Chem. 2015, 172, 543–550. [Google Scholar] [CrossRef] [PubMed]
- Balbach, J.K.; Royer, J.; Rogers, R. The effect of ownership on contract structure, costs, and quality: The case of the US beet sugar industry. In the Industrialization of Agriculture: Vertical Coordination in the US Food System; Royer, J.S., Rogers, R.T., Eds.; Ashgate Pub Ltd.: Farnham, UK, 1998; pp. 155–184. [Google Scholar]
- Roukas, T. Ethanol production from non-sterilized beet molasses by free and immobilized Saccharomyces cerevisiae cells using fed-batch culture. J. Food Eng. 1996, 27, 87–96. [Google Scholar] [CrossRef]
- Filipčev, B.; Lević, L.; Bodroža-Solarov, M.; Mišljenović, N.; Koprivica, G. Quality characteristics and antioxidant properties of breads supplemented with sugar beet molasses-based ingredients. Int. J. Food Prop. 2010, 13, 1035–1053. [Google Scholar] [CrossRef]
- Jiménez, A.M.; Borja, R.; Martın, A. Aerobic–anaerobic biodegradation of beet molasses alcoholic fermentation wastewater. Process. Biochem. 2003, 38, 1275–1284. [Google Scholar] [CrossRef]
- Obata, Y.; Senba, Y.; Koshika, M. Detection of phenolic compounds by chromatography in beet sugar molasses. Agric. Biol. Chem. 1963, 27, 340–341. [Google Scholar] [CrossRef]
- Valli, V.; Gómez-Caravaca, A.M.; di Nunzio, M.; Danesi, F.; Caboni, M.F.; Bordoni, A. Sugar cane and sugar beet molasses, antioxidant-rich alternatives to refined sugar. J. Agric. Food Chem. 2012, 60, 12508–12515. [Google Scholar] [CrossRef] [PubMed]
- Miles, E.A.; Zoubouli, P.; Calder, P.C. Differential anti-inflammatory effects of phenolic compounds from extra virgin olive oil identified in human whole blood cultures. Nutrition 2005, 21, 389–394. [Google Scholar] [CrossRef] [PubMed]
- Asikin, Y.; Takahashi, M.; Mishima, T.; Mizu, M.; Takara, K.; Wada, K. Antioxidant activity of sugarcane molasses against 2,2′-azobis(2-amidinopropane) dihydrochloride-induced peroxyl radicals. Food Chem. 2013, 141, 466–472. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.-J.; Yue, X.-F.; Han, J.-X.; Yang, W.-Y. Improved MTT assay for activity of antitumor agents. Chin. J. Pharm. 1993, 24, 455–457. [Google Scholar]
- Mukhtar, H.; Ahmad, N. Tea polyphenols: prevention of cancer and optimizing health. Am. J. Clin. Nutr. 2000, 71, 1698s–1702s. [Google Scholar] [PubMed]
- Elisia, I.; Kitts, D.D. Anthocyanins inhibit peroxyl radical-induced apoptosis in Caco-2 cells. Mol. Cell. Biochem. 2008, 312, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Forester, S.C.; Waterhouse, A.L. Gut metabolites of anthocyanins, gallic acid, 3-O-methylgallic acid, and 2,4,6-trihydroxybenzaldehyde, inhibit cell proliferation of Caco-2 cells. J. Agric. Food Chem. 2010, 58, 5320–5327. [Google Scholar] [CrossRef] [PubMed]
- Salucci, M.; Stivala, L.; Maiani, G.; Bugianesi, R.; Vannini, V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2). Br. J. Cancer 2002, 86, 1645–1651. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.M.; Bennett, R.N.; Needs, P.W.; Mellon, F.A.; Kroon, P.A.; Garcia-Conesa, M.-T. Characterization of metabolites of hydroxycinnamates in the in vitro model of human small intestinal epithelium Caco-2 cells. J. Agric. Food Chem. 2003, 51, 7884–7891. [Google Scholar] [CrossRef] [PubMed]
- Smets, L.A. Programmed cell death (apoptosis) and response to anti-cancer drugs. Anti-Cancer Drugs 1994, 5, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Sherr, C.J. Cancer cell cycles. Science 1996, 274, 1672–1677. [Google Scholar] [CrossRef] [PubMed]
- Blagosklonny, M.V. Cell cycle arrest is not senescence. Aging (Albany NY) 2011, 3, 94. [Google Scholar] [CrossRef] [PubMed]
- Wei, P.; Zhiyu, C.; Xu, T.; Xiangwei, Z. Antitumor effect and apoptosis induction of Alocasia cucullata (Lour.) G. Don in human gastric cancer cells in vitro and in vivo. BMC Complement. Altern. Med. 2015, 15, 33. [Google Scholar] [CrossRef] [PubMed]
- Donnellan, R.; Chetty, R. Cyclin D1 and human neoplasia. Mol. Pathol. 1998, 51, 1. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.; Sue, C.; Yu, C.; Chen, S.; Chen, G.; Chung, J. Diallyl disulfide (DADS) induced apoptosis undergo caspase-3 activity in human bladder cancer T24 cells. Food Chem. Toxicol. 2004, 42, 1543–1552. [Google Scholar] [CrossRef] [PubMed]
- Eastham, J.A.; Hall, S.J.; Sehgal, I.; Wang, J.; Timme, T.L.; Yang, G.; Connell-Crowley, L.; Elledge, S.J.; Zhang, W.-W.; Harper, J.W. In vivo gene therapy with p53 or p21 adenovirus for prostate cancer. Cancer Res. 1995, 55, 5151–5155. [Google Scholar] [PubMed]
- Schuyer, M.; van der Burg, M.; Henzen-Logmans, S.; Fieret, J.; Klijn, J.; Look, M.; Foekens, J.; Stoter, G.; Berns, E. Reduced expression of BAX is associated with poor prognosis in patients with epithelial ovarian cancer: A multifactorial analysis of TP53, p21, BAX and BCL-2. Br. J. Cancer 2001, 85, 1359. [Google Scholar] [CrossRef] [PubMed]






© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, M.; Zhao, Z.; Yu, S. Cytotoxicity and Apoptotic Effects of Polyphenols from Sugar Beet Molasses on Colon Carcinoma Cells in Vitro. Int. J. Mol. Sci. 2016, 17, 993. https://doi.org/10.3390/ijms17070993
Chen M, Zhao Z, Yu S. Cytotoxicity and Apoptotic Effects of Polyphenols from Sugar Beet Molasses on Colon Carcinoma Cells in Vitro. International Journal of Molecular Sciences. 2016; 17(7):993. https://doi.org/10.3390/ijms17070993
Chicago/Turabian StyleChen, Mingshun, Zhengang Zhao, and Shujuan Yu. 2016. "Cytotoxicity and Apoptotic Effects of Polyphenols from Sugar Beet Molasses on Colon Carcinoma Cells in Vitro" International Journal of Molecular Sciences 17, no. 7: 993. https://doi.org/10.3390/ijms17070993
APA StyleChen, M., Zhao, Z., & Yu, S. (2016). Cytotoxicity and Apoptotic Effects of Polyphenols from Sugar Beet Molasses on Colon Carcinoma Cells in Vitro. International Journal of Molecular Sciences, 17(7), 993. https://doi.org/10.3390/ijms17070993