Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening
Abstract
:1. Introduction
2. Results
2.1. Quality Traits of Apricot during Development and Ripening
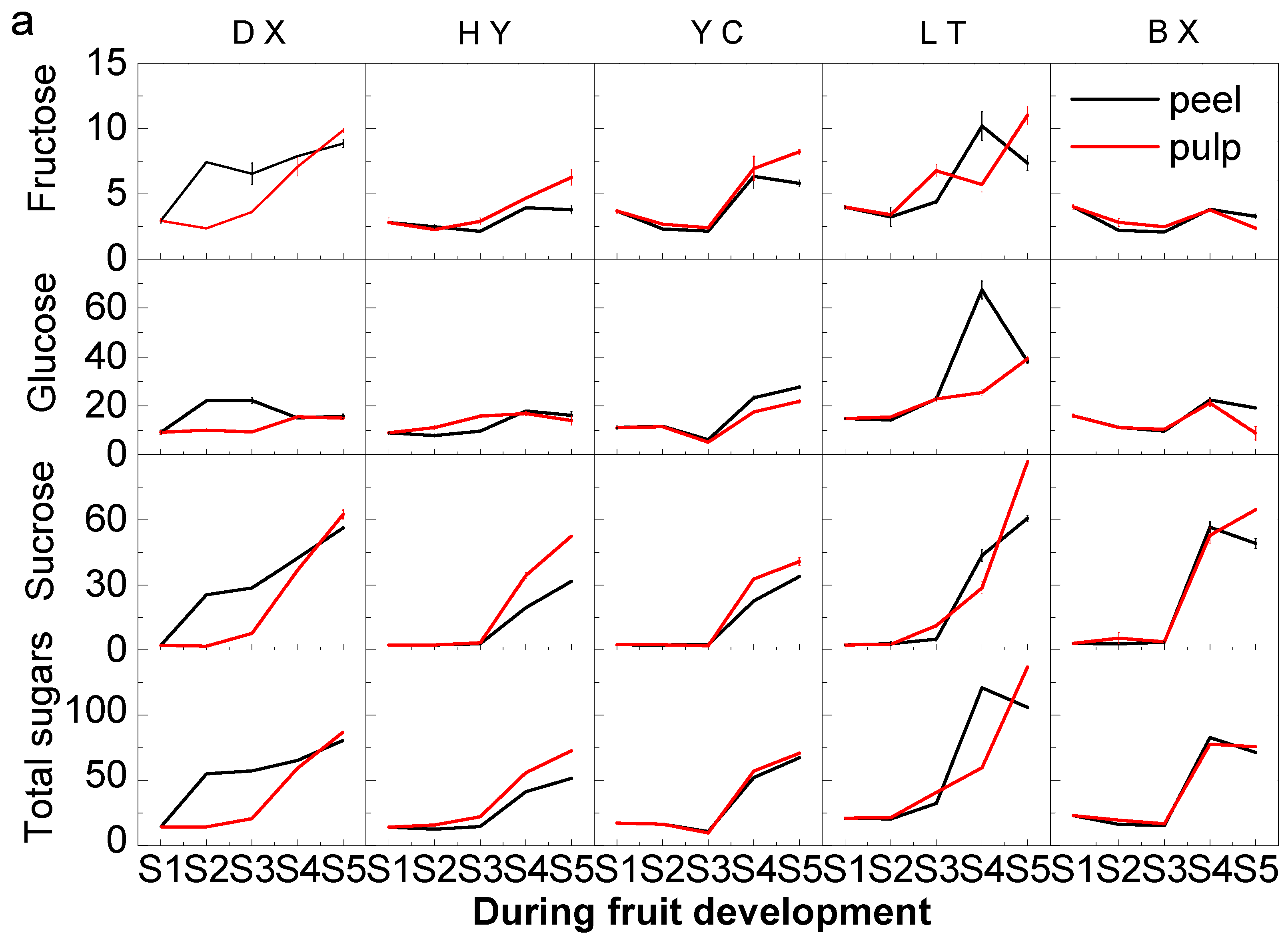
2.2. Changes in Sugars during Fruit Development and Ripening
2.3. Changes in Organic Acids during Fruit Development and Ripening
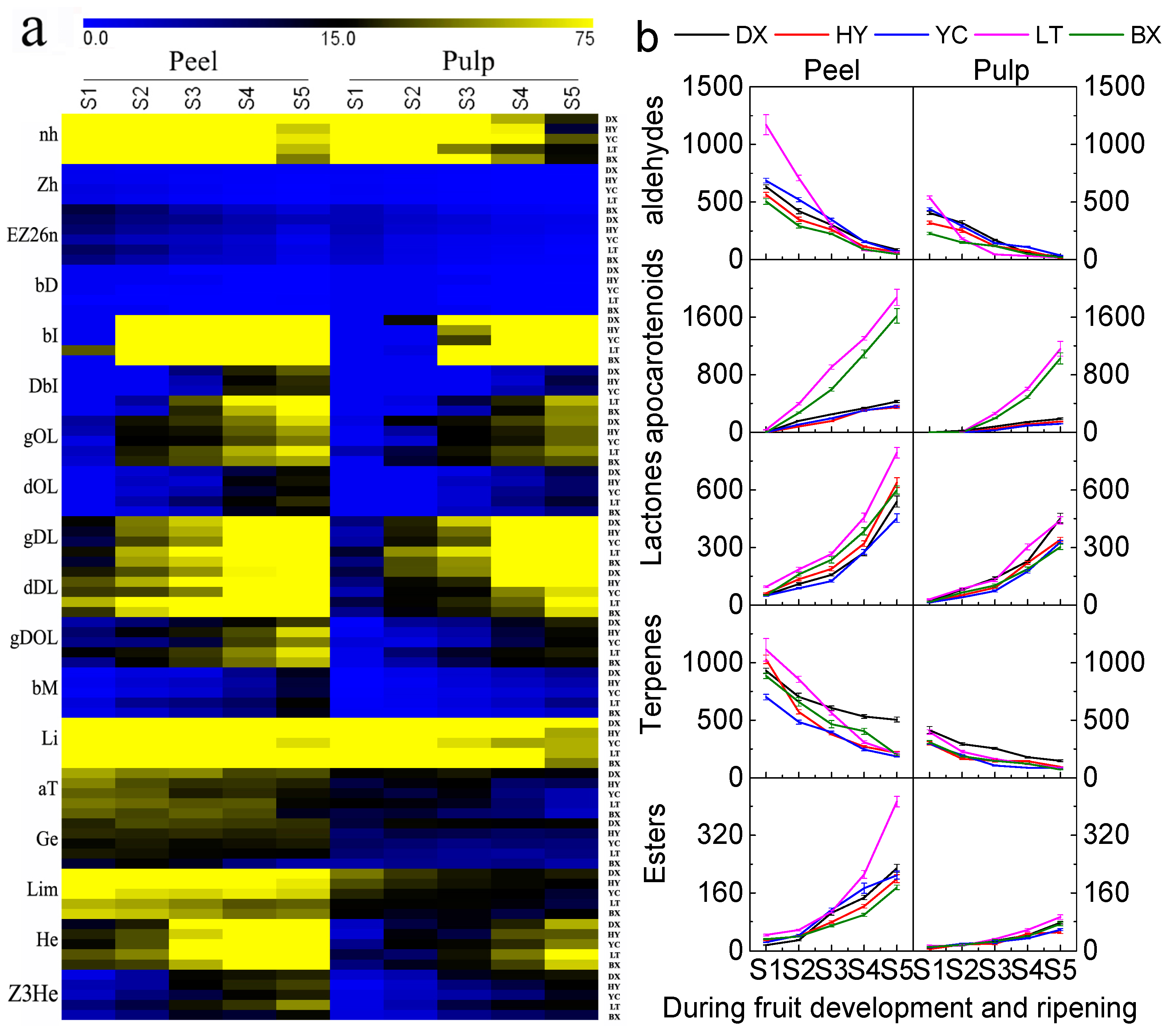
2.4. Changes in Aroma Volatiles during Development and Ripening
2.5. Effect of Flavor Compounds and Ripening on Consumer Acceptance
2.6. Enzymes Involved in Flavor Compound Metabolism during Development and Ripening
2.7. Correlation between Enzymatic Capacities and Flavor Compound Accumulation
3. Discussion
4. Materials and Methods
4.1. Fruit Materials Preparation
4.2. Fruit Quality Traits Determination
4.3. HPLC Analysis on Sugars and Organic Acids
4.4. GC-MS Analysis
4.5. Flavor and Consumer’s Acceptance Determinations
4.6. Determination of Enzymes Activities
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| TA | titratable acidity |
| TSS | total soluble solid |
| S1 | stage 1 |
| DX | danxing |
| HY | Hongyu |
| YC | Yechengheiyexing |
| LT | luntaixiaobaixing |
| BX | baixing |
| FW | fresh weight |
| OOT | orthonasal odor thresholds |
| OAV | odor activity values |
| PLSR | partial least squares regression |
| SSthy | sucrose synthase for synthesis direction |
| SPS | sucrose phosphatesynthase |
| SSca | sucrose synthase for degradation direction |
| NI | neutral invertase |
| AI | acid invertase |
| SDH | sorbitol dehydrogenase |
| FK | fructokinase |
| SO | sorbitol oxidase |
| GK | glucokinase |
| QH | quinate dehydrogenase |
| MS | malate synthase |
| NADP-ME | NADP-malic enzyme |
| NAD-ME | NAD-malic enzyme |
| CS | citrate synthase |
| GAD | glutamate decarboxylase |
| LOX | lipoxygenases |
| HPL | hydroperoxide lyase |
| ADH | alcohol dehydrogenase |
| AAT | alcohol acyl-transferases |
| ACX | Acyl-CoA Oxidase |
| CCD | carotenoid cleavage dioxygenases |
| TPS | terpene Synthase |
References
- Goff, S.A.; Klee, H.J. Plant volatile compounds: Sensory cues for health and nutritional value? Science 2006, 311, 815–819. [Google Scholar] [CrossRef] [PubMed]
- Kader, A.A. Flavor quality of fruits and vegetables. J. Sci. Food Agric. 2008, 88, 1863–1868. [Google Scholar] [CrossRef]
- Klee, H.J. Improving the flavor of fresh fruits: Genomics, biochemistry, and biotechnology. New Phytol. 2010, 187, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Desnoues, E.; Gibon, Y.; Baldazzi, V.; Signoret, V.; Génard, M.; Quilot-Turion, B. Profiling sugar metabolism during fruit development in a peach progeny with different fructose-to-glucose ratios. BMC Plant Biol. 2014, 14, 336. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.-X.; Liu, X.-H.; Chen, L.-S. Developmental changes in pulp organic acid concentration and activities of acid-metabolising enzymes during the fruit development of two loquat (Eriobotrya. japonica Lindl.) cultivars differing in fruit acidity. Food Chem. 2009, 114, 657–664. [Google Scholar] [CrossRef]
- Moing, A.; Renaud, C.; Gaudillère, M.; Raymond, P.; Roudeillac, P.; Denoyes-Rothan, B. Biochemical changes during fruit development of four strawberry cultivars. J. Am. Soc. Hortic. Sci. 2001, 126, 394–403. [Google Scholar]
- Oms-Oliu, G.; Hertog, M.; van de Poel, B.; Ampofo-Asiama, J.; Geeraerd, A.; Nicolaï, B. Metabolic characterization of tomato fruit during preharvest development, ripening, and postharvest shelf-life. Postharvest Biol. Technol. 2011, 62, 7–16. [Google Scholar] [CrossRef]
- Borsani, J.; Budde, C.O.; Porrini, L.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F.; Lara, M.V. Carbon metabolism of peach fruit after harvest: changes in enzymes involved in organic acid and sugar level modifications. J. Exp. Bot. 2009, 60, 1823–1837. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, V.A.; Osorio, S.; Borsani, J.; Lauxmann, M.A.; Bustamante, C.A.; Budde, C.O.; Andreo, C.S.; Lara, M.V.; Fernie, A.R.; Drincovich, M.F. Metabolic profiling during peach fruit development and ripening reveals the metabolic networks that underpin each developmental stage. Plant Physiol. 2011, 157, 1696–1710. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.P.; Singh, Z.; Swinny, E.E. Sugars and organic acids in Japanese plums (Prunus salicina Lindell) as influenced by maturation, harvest date, storage temperature and period. Int. J. Food Sci. Technol. 2009, 44, 1973–1982. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, X.; Yu, O.; Tang, J.; Gu, X.; Wan, X.; Fang, C. Metabolic profiling of strawberry (Fragaria × ananassa Duch.) during fruit development and maturation. J. Exp. Bot. 2010. [Google Scholar] [CrossRef]
- Basson, C.; Groenewald, J.; Kossmann, J.; Cronjé, C.; Bauer, R. Sugar and acid-related quality attributes and enzyme activities in strawberry fruits: Invertase is the main sucrose hydrolysing enzyme. Food Chem. 2010, 121, 1156–1162. [Google Scholar] [CrossRef]
- Yu, K.; Xu, Q.; Da, X.; Guo, F.; Ding, Y.; Deng, X. Transcriptome changes during fruit development and ripening of sweet orange (Citrus sinensis). BMC Genom. 2012, 13, 1. [Google Scholar] [CrossRef] [PubMed]
- Tang, M.; Bie, Z.-L.; Wu, M.-Z.; Yi, H.-P.; Feng, J.-X. Changes in organic acids and acid metabolism enzymes in melon fruit during development. Sci. Hortic. 2010, 123, 360–365. [Google Scholar] [CrossRef]
- Saradhuldhat, P.; Paull, R.E. Pineapple organic acid metabolism and accumulation during fruit development. Sci. Hortic. 2007, 112, 297–303. [Google Scholar] [CrossRef]
- Kalua, C.M.; Boss, P.K. Evolution of volatile compounds during the development of Cabernet Sauvignon grapes (Vitis. vinifera L.). J. Agric. Food Chem. 2009, 57, 3818–3830. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, G.; Cam, M.; Kutlu, T.; HIşIL, Y. Some physical and chemical changes during fruit development of five common apricot (Prunus armeniaca L.) cultivars. Food Sci. Technol. Res. 2010, 16, 71–78. [Google Scholar] [CrossRef]
- González-Agüero, M.; Troncoso, S.; Gudenschwager, O.; Campos-Vargas, R.; Moya-León, M.A.; Defilippi, B.G. Differential expression levels of aroma-related genes during ripening of apricot (Prunus armeniaca L.). Plant. Physiol. Biochem. 2009, 47, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Bureau, S.; Ruiz, D.; Reich, M.; Gouble, B.; Bertrand, D.; Audergon, J.-M.; Renard, C.M. Application of ATR-FTIR for a rapid and simultaneous determination of sugars and organic acids in apricot fruit. Food Chem. 2009, 115, 1133–1140. [Google Scholar] [CrossRef]
- Wu, B.; Quilot, B.; Génard, M.; Kervella, J.; Li, S. Changes in sugar and organic acid concentrations during fruit maturation in peaches, P. davidiana and hybrids as analyzed by principal component analysis. Sci. Hortic. 2005, 103, 429–439. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, P.; Cheng, L. Developmental changes of carbohydrates, organic acids, amino acids, and phenolic compounds in ′Honeycrisp′ apple flesh. Food Chem. 2010, 123, 1013–1018. [Google Scholar] [CrossRef]
- Medlicott, A.P.; Thompson, A.K. Analysis of sugars and organic acids in ripening mango fruits (Mangifera. indica L. var Keitt) by high performance liquid chromatography. J. Sci. Food Agric. 1985, 36, 561–566. [Google Scholar] [CrossRef]
- Glew, R.H.; Ayaz, F.A.; Millson, M.; Huang, H.; Chuang, L.; Sanz, C.; Golding, J.B. Changes in sugars, acids and fatty acids in naturally parthenocarpic date plum persimmon (Diospyros. lotus L.) fruit during maturation and ripening. Eur. Food Res. Technol. 2005, 221, 113–118. [Google Scholar] [CrossRef]
- Li, M.; Feng, F.; Cheng, L. Expression patterns of genes involved in sugar metabolism and accumulation during apple fruit development. PLoS ONE 2012, 7, e33055. [Google Scholar] [CrossRef] [PubMed]
- Moing, A.; Svanella, L.; Rolin, D.; Gaudillère, M.; Gaudillère, J.-P.; Monet, R. Compositional changes during the fruit development of two peach cultivars differing in juice acidity. J. Am. Soc. Hortic. Sci. 1998, 123, 770–775. [Google Scholar]
- Albertini, M.-V.; Carcouet, E.; Pailly, O.; Gambotti, C.; Luro, F.; Berti, L. Changes in organic acids and sugars during early stages of development of acidic and acidless citrus fruit. J. Agric. Food Chem. 2006, 54, 8335–8339. [Google Scholar] [CrossRef] [PubMed]
- Verde, I.; Abbott, A.G.; Scalabrin, S.; Jung, S.; Shu, S.; Marroni, F.; Zhebentyayeva, T.; Dettori, M.T.; Grimwood, J.; Cattonaro, F. The high-quality draft genome of peach (Prunus persica) identifies unique patterns of genetic diversity, domestication and genome evolution. Nat. Genet. 2013, 45, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Etienne, C.; Moing, A.; Dirlewanger, E.; Raymond, P.; Monet, R.; Rothan, C. Isolation and characterization of six peach cDNAs encoding key proteins in organic acid metabolism and solute accumulation: Involvement in regulating peach fruit acidity. Physiol. Plant. 2002, 114, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Greger, V.; Schieberle, P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J. Agric. Food Chem. 2007, 55, 5221–5228. [Google Scholar] [CrossRef] [PubMed]
- Lo Bianco, R.; Farina, V.; Indelicato, S.G.; Filizzola, F.; Agozzino, P. Fruit physical, chemical and aromatic attributes of early, intermediate and late apricot cultivars. J. Sci. Food Agric. 2010, 90, 1008–1019. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Shen, J.-Y.; Wei, W.-W.; Xi, W.-P.; Xu, C.-J.; Ferguson, I.; Chen, K. Expression of genes associated with aroma formation derived from the fatty acid pathway during peach fruit ripening. J. Agric. Food Chem. 2010, 58, 6157–6165. [Google Scholar] [CrossRef] [PubMed]
- Xi, W.; Zhang, Q.; Lu, X.; Wei, C.; Yu, S.; Zhou, Z. Improvement of flavour quality and consumer acceptance during postharvest ripening in greenhouse peaches by carbon dioxide enrichment. Food Chem. 2014, 164, 219–227. [Google Scholar] [CrossRef] [PubMed]
- Lewinsohn, E.; Sitrit, Y.; Bar, E.; Azulay, Y.; Ibdah, M.; Meir, A.; Yosef, E.; Zamir, D.; Tadmor, Y. Not just colors—Carotenoid degradation as a link between pigmentation and aroma in tomato and watermelon fruit. Trends Food Sci. Technol. 2005, 16, 407–415. [Google Scholar] [CrossRef]
- Walter, M.H.; Floss, D.S.; Strack, D. Apocarotenoids: Hormones, mycorrhizal metabolites and aroma volatiles. Planta 2010, 232, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, K.; Zhang, B.; Sun, C.; Cai, C.; Zhou, C.; Xu, W.; Zhang, W.; Ferguson, I.B. Postharvest responses of Chinese bayberry fruit. Postharvest Biol. Technol. 2005, 37, 241–251. [Google Scholar] [CrossRef]
- Eduardo, I.; Chietera, G.; Bassi, D.; Rossini, L.; Vecchietti, A. Identification of key odor volatile compounds in the essential oil of nine peach accessions. J. Sci. Food Agric. 2010, 90, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Altisent, R.; Graell, J.; Lara, I.; Lopez, L.; Echeverria, G. Regeneration of volatile compounds in Fuji apples following ultra low oxygen atmosphere storage and its effect on sensory acceptability. J. Agric. Food Chem. 2008, 56, 8490–8497. [Google Scholar] [CrossRef] [PubMed]
- Lara, M.V.; Borsani, J.; Budde, C.O.; Lauxmann, M.A.; Lombardo, V.A.; Murray, R.; Andreo, C.S.; Drincovich, M.F. Biochemical and proteomic analysis of ‘Dixiland’peach fruit (Prunus persica) upon heat treatment. J. Exp. Bot. 2009. [Google Scholar] [CrossRef] [PubMed]
- Van Kleef, M.; Duine, J. Bacterial NAD(P)-independent quinate dehydrogenase is a quinoprotein. Arch. Microb. 1988, 150, 32–36. [Google Scholar] [CrossRef]
- Snedden, W.A.; Arazi, T.; Fromm, H.; Shelp, B.J. Calcium/calmodulin activation of soybean glutamate decarboxylase. Plant Physiol. 1995, 108, 543–549. [Google Scholar] [PubMed]
- Ortiz, A.; Echeverría, G.; Graell, J.; Lara, I. Overall quality of 'Rich Lady′ peach fruit after air- or CA storage. The importance of volatile emission. LWT-Food Sci. Technol. 2009, 42, 1520–1529. [Google Scholar] [CrossRef]
- Abbott, E.; Hall, D.; Hamberger, B.; Bohlmann, J. Laser microdissection of conifer stem tissues: Isolation and analysis of high quality RNA, terpene synthase enzyme activity and terpenoid metabolites from resin ducts and cambial zone tissue of white spruce (Picea glauca). BMC Plant Biol. 2010, 10, 106. [Google Scholar] [CrossRef] [PubMed]








| No. | Cultivars | Abbreviation | Repository No. 1 | Color Character | DAB 2 | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| peel | pulp | S1 | S2 | S3 | S4 | S5 | ||||
| 1 | Danxing | DX | XD076 | red | orange | 21 | 32 | 56 | 74 | 82 |
| 2 | Hongyuxing | HY | XD117 | red | orange | 21 | 32 | 61 | 74 | 82 |
| 3 | Yechengheiyexing | YC | XD018 | orange | white | 21 | 27 | 61 | 91 | 91 |
| 4 | Luntaixiaobaixing | LT | XD021 | white | light yellow | 21 | 27 | 57 | 65 | 74 |
| 5 | Baixing | BX | XD094 | white | white | 21 | 27 | 61 | 88 | 91 |
| No. | Aroma Compound | Code | OOT 1 | Aroma Quality | OAV 2 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Peel | Puip | |||||||||||||
| DX | HY | YC | LT | BX | DX | HY | YC | LT | BX | |||||
| 1 | hexanal | nh | 2.4 | Green, grassy | 34.03 | 25.97 | 29.03 | 24.72 | 18.33 | 10.56 | 4.86 | 14.86 | 7.50 | 8.19 |
| 2 | (Z)-3-hexenal | Zh | 0.12 | Green, grassy | 5.42 | 3.17 | 2.58 | 1.75 | 19.75 | 1.33 | 0.92 | 1.17 | 1.17 | 5.42 |
| 3 | (E,Z)-2,6-nonadienal | EZ26n | 0.03 | Cucumber-like | 148.33 | 78 | 39 | 59.33 | 48.33 | 49 | 41 | 21.67 | 14.33 | 18.67 |
| 4 | β-damascenone | bD | 0.002 | Baked apple-like, grape juice-like | 45 | 40 | 55 | 180 | 145 | 26 | 35 | 15 | 130 | 85 |
| 5 | β-ionone | bI | 3.5 | Flowery, violet-like | 111 | 91.43 | 97.14 | 506 | 434 | 51.43 | 40 | 31.43 | 314 | 280 |
| 6 | dihydro-β-ionone | DbI | 5 | Fresh rosy note | 7.40 | 5.20 | 5.01 | 20.80 | 19.40 | 1.60 | 2.20 | 1.80 | 11.60 | 9.20 |
| 7 | γ-octalactone | gOL | 6.5 | Coconut-like | 10.31 | 8.12 | 6.62 | 10.92 | 8.31 | 7.23 | 5.85 | 5.85 | 7.23 | 5.23 |
| 8 | δ-octalactone | dOL | 0.4 | Coconut-like, sweet odor | 47.50 | 52.50 | 40 | 65 | 42.50 | 27.50 | 22.50 | 22.50 | 30 | 20 |
| 9 | γ-decalactone | gDL | 1.1 | Peach-like, coconut-like | 281 | 210 | 155 | 319 | 213 | 225 | 146 | 181 | 252 | 160 |
| 10 | δ-decalactone | dDL | 53 | Coconut-like | 2.16 | 5.04 | 3.36 | 5.21 | 4.42 | 2.32 | 2.17 | 1.26 | 1.55 | 1.26 |
| 11 | γ-dodecalactone | gDOL | 0.43 | Peach-like | 66.57 | 155 | 103 | 160 | 136 | 55.81 | 44.19 | 37.21 | 52.33 | 41.86 |
| 12 | β-myrcene | bM | 1.2 | Hop-like, geranium-like | 10.83 | 9.67 | 9.33 | 13.67 | 11.17 | 5.83 | 4.33 | 3.17 | 6.17 | 3 |
| 13 | linalool | Li | 6 | Citrus-like, bergamot-like | 57.50 | 14.50 | 11.17 | 20.67 | 20.67 | 14.50 | 9 | 9.33 | 9.33 | 7.50 |
| 14 | α-terpineol | aT | 0.33 | Lilac-like, peach-like | 103 | 72.73 | 60.61 | 54.55 | 39.39 | 42.42 | 30.30 | 15.15 | 15.15 | 12.12 |
| 15 | geraniol | Ge | 1.1 | Rose-like, citrus-like | 24.55 | 23.18 | 20.45 | 14.09 | 5 | 15.46 | 9.09 | 7.27 | 6.82 | 5 |
| 16 | limonene | Lim | 10 | Citrus-like | 8.60 | 7 | 6.50 | 3.80 | 4.5 | 2.3 | 1.5 | 1.2 | 1.1 | 1.5 |
| 17 | hexyl acetate | He | 2 | A mild sweet odor | 102 | 84 | 93 | 183 | 78 | 28 | 18 | 22.50 | 38 | 32 |
| 18 | (Z)-3-hexenyl acetate | Z3He | 3.9 | Green; banana-like | 6.15 | 7.95 | 5.90 | 12 | 4.9 | 5.4 | 3.9 | 3.3 | 4.4 | 2.8 |
| Sensory Attributes | S4 | S5 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DX | HY | YC | LT | BX | DX | HY | YC | LT | BX | |
| Sweetness | 5.61 | 4.93 | 4.82 | 6.87 | 6.14 | 8.42 | 6.78 | 6.23 | 8.77 | 7.53 |
| Sourness | 5.49 | 4.46 | 4.23 | 4.92 | 4.36 | 3.44 | 3.82 | 2.17 | 2.54 | 3.36 |
| Aroma | 6.21 | 5.78 | 5.24 | 7.36 | 6.89 | 7.63 | 6.25 | 6.84 | 8.79 | 8.13 |
| Flavor | 6.54 | 5.08 | 5.57 | 7.54 | 7.21 | 7.91 | 6.45 | 6.22 | 8.56 | 7.81 |
| Acceptability | 6.93 | 5.74 | 5.36 | 8.12 | 7.76 | 8.13 | 7.17 | 7.60 | 9.03 | 8.42 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xi, W.; Zheng, H.; Zhang, Q.; Li, W. Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening. Int. J. Mol. Sci. 2016, 17, 998. https://doi.org/10.3390/ijms17070998
Xi W, Zheng H, Zhang Q, Li W. Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening. International Journal of Molecular Sciences. 2016; 17(7):998. https://doi.org/10.3390/ijms17070998
Chicago/Turabian StyleXi, Wanpeng, Huiwen Zheng, Qiuyun Zhang, and Wenhui Li. 2016. "Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening" International Journal of Molecular Sciences 17, no. 7: 998. https://doi.org/10.3390/ijms17070998
APA StyleXi, W., Zheng, H., Zhang, Q., & Li, W. (2016). Profiling Taste and Aroma Compound Metabolism during Apricot Fruit Development and Ripening. International Journal of Molecular Sciences, 17(7), 998. https://doi.org/10.3390/ijms17070998






