Characterization and Discrimination of Ancient Grains: A Metabolomics Approach
Abstract
:1. Introduction
2. Results
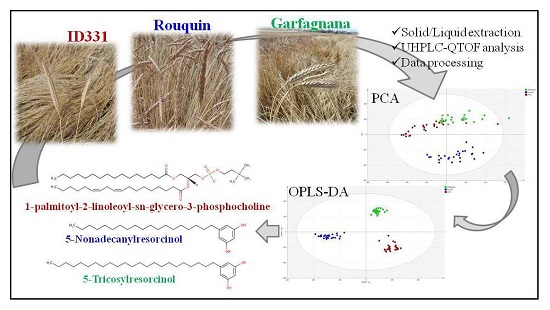
2.1. Multivariate Modeling
2.2. Discriminant Metabolites Identification
3. Discussion
3.1. Phenolic Compounds
3.2. Glycerophospholipids and Glycerolipids
4. Materials and Methods
4.1. Chemicals
4.2. Plant Material
4.3. Metabolite Extraction
4.4. Quality Control (QC) Samples Preparation
4.5. Ultra-High-Performance Liquid Chromatography-High Resolution Mass Spectrometry
4.6. Data Processing and Chemometrics Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| ARs | alkylresorcinols |
| QTOF | quadrupole time of flight |
| HRMS | high resolution mass spectrometer |
| QC | quality control |
| PCA | principal component analysis |
| PLS-DA | projection on latent structure-discriminant analysis |
| OPLS-DA | orthogonal projection on latent structure-discriminant analysis |
| DG | diacylglycerol |
| TG | triacylglycerol |
| PC | phosphatidylcholine |
| Lyso PC | lysophosphatidylcholine |
References
- Shewry, P.R. Wheat. J. Exp. Bot. 2009, 60, 1537–1553. [Google Scholar] [CrossRef] [PubMed]
- Serpen, A.; Gokmen, V.; Karagoz, A.; Koksel, H. Phytochemical quantification and total antioxidant capacities of Emmer (Triticum dicoccon Schrank) and Einkorn (Triticum monococcum L.) wheat landraces. J. Agric. Food Chem. 2008, 56, 7285–7292. [Google Scholar] [CrossRef] [PubMed]
- Galterio, G.; Codianni, P.; Giusti, A.M.; Pezzarossa, B.; Cannella, C. Assessment of the agronomic and technological characteristics of Triticum turgidum ssp. dicoccum Schrank and T. spelta L. Nahrung 2003, 47, 54–59. [Google Scholar]
- Hidalgo, A.; Brandolini, A. Nutritional properties of einkorn wheat (Triticum monococcum L.). J. Sci. Food Agric. 2014, 94, 601–612. [Google Scholar] [CrossRef] [PubMed]
- Bonafaccia, G.; Galli, V.; Francisci, R.; Mair, V.; Skrabanja, V.; Kreft, I. Characteristics of spelt wheat products and nutritional value of spelt wheat-based bread. Food Chem. 2000, 68, 437–441. [Google Scholar] [CrossRef]
- Buvaneshwari, G.; Yenagi, N.B.; Hanchinal, R.R.; Naik, R.K. Glycaemic responses to dicoccum products in the dietary management of diabetes. Ind. J. Nutr. Diet. 2003, 40, 363–368. [Google Scholar]
- Piergiovanni, A.R.; Rizzi, R.; Pannaciulli, E.; Della Gatta, C. Mineral composition in hulled wheat grains: A comparison between emmer (Triticum dicoccon Schrank) and spelt (T. spelta L.) accessions. Int. J. Food Sci. Nutr. 1997, 48, 381–386. [Google Scholar] [CrossRef]
- Landberg, R.; Kamal-Eldin, A.; Salmenkallio-Marttila, M.; Rouau, X.; Åman, P. Localization of alkylresorcinols in wheat, rye and barley kernels. J. Cereal Sci. 2008, 48, 401–406. [Google Scholar] [CrossRef]
- Knodler, M.; Most, M.; Schieber, A.; Carle, R. A novel approach to authenticity control of whole grain durum wheat (Triticum durum Desf.) flour and pasta, based on analysis of alkylresorcinol composition. Food Chem. 2010, 118, 177–181. [Google Scholar] [CrossRef]
- Ziegler, J.U.; Steingass, C.B.; Longin, C.F.; Würschum, T.; Reinhold, C.; Schweiggert, R.M. Alkylresorcinol composition allows the differentiation of Triticum spp. having different degrees of ploidy. J. Cereal Sci. 2015, 65, 244–251. [Google Scholar] [CrossRef]
- Rubert, J.; Zachariasova, M.; Hajslova, J. Advances in high-resolution mass spectrometry based on metabolomics studies for food—A review. Food Addit. Contam. Part A 2015, 32, 1685–1708. [Google Scholar] [CrossRef] [PubMed]
- Rubert, J.; Lacina, O.; Fauhl-Hassek, C.; Hajslova, J. Metabolic fingerprinting based on high-resolution tandem mass spectrometry: A reliable tool for wine authentication? Anal. Bioanal. Chem. 2014, 406, 6791–6803. [Google Scholar] [CrossRef] [PubMed]
- Matthews, S.B.; Santra, M.; Mensack, M.M.; Wolfe, P.; Byrne, P.F.; Thompson, H.J. Metabolite profiling of a diverse collection of wheat lines using ultraperformance liquid chromatography coupled with time-of-flight mass spectrometry. PLoS ONE 2012, 7, e44179. [Google Scholar] [CrossRef] [PubMed]
- Rubingh, C.M.; Bijlsma, S.; Derks, E.; Bobeldijk, I.; Verheij, E.R. Assessing the performance of statistical validation tools for megavariate metabolomics data. Metabolomics 2006, 2, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Holcapek, M.; Jandera, P.; Zderadicka, P.; Hruba, L. Characterization of triacylglycerol and diacylglycerol composition of plant oils using high-performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J. Chromatogr. A 2003, 1010, 195–215. [Google Scholar] [CrossRef]
- Bird, S.S.; Marur, V.R.; Sniatynski, M.J.; Greenberg, H.K.; Kristal, B.S. Serum lipidomics profiling using LC-MS and high-energy collisional dissociation fragmentation: Focus on triglyceride detection and characterization. Anal. Chem. 2011, 83, 6648–6657. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Kamal-Eldin, A.; Jung, C.; Shepherd, M.J.; Åman, P. Gas chromatographic analysis of alkylresorcinols in rye (Secale cereale L.) grains. J. Agric. Food Chem. 2001, 81, 1405–1411. [Google Scholar] [CrossRef]
- Kulawinek, M.; Kozubek, A. Quantitative determination of alkylresorcinols in cereal grains: Independence of the length of the aliphatic side chain. J. Food Lipids 2008, 15, 251–262. [Google Scholar] [CrossRef]
- Chen, J.; He, S.; Mao, H.; Sun, C.; Pan, Y. Characterization of polyphenol compounds from the roots and stems of Parthenocissus laetevirens by high-performance liquid chromatography/tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2009, 23, 737–744. [Google Scholar] [CrossRef] [PubMed]
- Milne, S.; Ivanova, P.; Forrester, J.; Brown, A.H. Lipidomics: An analysis of cellular lipids by ESI-MS. Methods 2006, 39, 92–103. [Google Scholar] [CrossRef] [PubMed]
- Andersson, A.M.; Kamal-Eldin, A.; Fras, A.; Boros, D.; Amam, P. Alkylresorcinols in wheat varieties in the HEALTHGRAIN diversity screen. J. Agric. Food Chem. 2008, 56, 9722–9725. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Shepherd, M.J.; Schüpphaus, M.; Sinclair, V.; Alfaro, B.; Kamal-Eldin, A.; Åman, P. Alkylresorcinols in cereals and cereal products. J. Agric. Food Chem. 2003, 51, 4111–4118. [Google Scholar] [CrossRef] [PubMed]
- Kozubek, A.; Tyman, J.H.P. Resorcinolic lipids, the natural non-isoprenoid phenolic amphiphiles and their biological activity. Chem. Rev. 1999, 99, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Andersson, U.; Dey, E.S.; Holm, C.; Degerman, E. Rye bran alkylresorcinols suppress adipocyte lipolysis and hormone-sensitive lipase activity. Mol. Nutr. Food Res. 2011, 55, S290–S293. [Google Scholar] [CrossRef] [PubMed]
- Ross, A.B.; Shepherd, M.J.; Knudsen, K.E.B.; Glitsø, L.V.; Bowey, E.; Phillips, J.; Rowland, I.; Guo, Z.X.; Massy, D.J.R.; Aman, P.; et al. Absorption of dietary alkylresorcinols in ileal-cannulated pigs and rats. Br. J. Nutr. 2003, 90, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Hanhineva, K.; Brunius, C.; Andersson, A.; Marklund, M.; Juvonen, R.; Keski-Rahkonen, P.; Auriola, S.; Landberg, R. Discovery of urinary biomarkers of whole grain rye intake in free-living subjects using nontargeted LC-MS metabolite profiling. Mol. Nutr. Food Res. 2015, 59, 2315–2325. [Google Scholar] [CrossRef] [PubMed]
- Zarnowski, R.; Suzuki, Y.; Pietr, S.J. Alkyl- and alkenylresorcinols of wheat grains and their chemotaxonomic significance. Z. Naturforsch. C 2004, 59, 190–196. [Google Scholar] [CrossRef] [PubMed]
- González-Thuillier, I.; Salt, L.; Chope, G.; Penson, S.; Skeggs, P.; Tosi, P.; Powers, S.J.; Ward, J.L.; Wilde, P.; Shewry, P.R.; et al. Distribution of lipids in the grain of wheat (cv. Hereward) determined by lipidomic analysis of milling and pearling fractions. J. Agric. Food Chem. 2015, 63, 10705–10710. [Google Scholar] [CrossRef] [PubMed]




| Statistical Parameters | ESI(+) Models | ESI(−) Models | ||||
|---|---|---|---|---|---|---|
| PCA | PLS-DA | OPLS-DA | PCA | PLS-DA | OPLS-DA | |
| R2X (cum) | 0.816 | 0.578 | 0.579 | 0.89 | 0.709 | 0.709 |
| R2Y (cum) | - | 0.942 | 0.942 | - | 0.967 | 0.967 |
| Q2 (cum) | 0.663 | 0.916 | 0.917 | 0.778 | 0.944 | 0.956 |
| Biochemical Category | Biochemical Class | Tentative Identification | Pseudomolecolar Ion | m/z | RT (min) | Elemental Formula | Mass Error (Δppm) | Higher Metabolite Intensity in | VIP Value |
|---|---|---|---|---|---|---|---|---|---|
| Phenols | Resorcinols | Heptadecylresorcinol (C17:0) | [M − H]− | 347.2956 | 6.3 | C23H40O2 | 1.7 | spelt | 1.5 |
| Nonadecanylresorcinol (C19:0) | [M − H]− | 375.3269 | 6.9 | C25H44O2 | 1.5 | spelt | 4.2 | ||
| Nonadecenyl-resorcinol (C19:1) | [M − H]− | 373.3112 | 6.3 | C25H42O2 | 1.4 | spelt | 2.2 | ||
| Heneicosylresorcinol (C21:0) | [M − H]− | 403.3582 | 7.4 | C27H48O2 | 1.4 | spelt | 2.9 | ||
| Heneicosenyl-resorcinol (C21:1) | [M − H]− | 401.3425 | 6.9 | C27H46O2 | 1.3 | spelt | 1.5 | ||
| Tricosylresorcinol (C23:0) | [M − H]− | 431.3895 | 8 | C29H52O2 | 1.3 | emmer | 3.2 | ||
| Pentacosylresorcinol (C25:0) | [M − H]− | 459.4208 | 8.5 | C31H56O2 | 1.2 | emmer | 3.1 | ||
| Glycerophospholipids (GLP) | Lysophosphatidylcholines (LysoPC) | LysoPC 16:0 | [M + H]+ | 496.3399 | 4.5 | C24H50NO7P | 3.2 | einkorn | 4.3 |
| LysoPC 18:2 | [M + H]+ | 520.3392 | 4.2 | C26H50NO7P | 1.2 | einkorn | 3.1 | ||
| Phosphatidylcholines (PC) | PC 16:0/18:1 | [M + H]+ | 760.5851 | 8.2 | C42H82O8NP | 1.6 | einkorn | 2.9 | |
| PC 16:0/18:2 | [M + H]+ | 758.5712 | 7.9 | C42H80NO8P | 2.3 | einkorn | 3.9 | ||
| Phosphatidylinositols (PI) | PI 16:0/18:1 | [M + H]+ | 835.5478 | 7.7 | C43H81O13P | 1.6 | emmer | 1.6 | |
| Glycerolipids (GL) | Diacylglycerols (DG) | DG 16:0/18:2 | [M + NH4]+ | 610.5405 | 8.8 | C37H68O5 | 1.5 | emmer | 4.9 |
| Triacylglycerols (TG) | TG 16:0/16:0/18:2 | [M + NH4]+ | 848.7708 | 11.4 | C53H98O6 | 1.8 | spelt | 3.1 | |
| TG 16:0/18:1/20:1 | [M + NH4]+ | 904.8339 | 12 | C57H106O6 | 1.9 | einkorn | 3.8 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Righetti, L.; Rubert, J.; Galaverna, G.; Folloni, S.; Ranieri, R.; Stranska-Zachariasova, M.; Hajslova, J.; Dall’Asta, C. Characterization and Discrimination of Ancient Grains: A Metabolomics Approach. Int. J. Mol. Sci. 2016, 17, 1217. https://doi.org/10.3390/ijms17081217
Righetti L, Rubert J, Galaverna G, Folloni S, Ranieri R, Stranska-Zachariasova M, Hajslova J, Dall’Asta C. Characterization and Discrimination of Ancient Grains: A Metabolomics Approach. International Journal of Molecular Sciences. 2016; 17(8):1217. https://doi.org/10.3390/ijms17081217
Chicago/Turabian StyleRighetti, Laura, Josep Rubert, Gianni Galaverna, Silvia Folloni, Roberto Ranieri, Milena Stranska-Zachariasova, Jana Hajslova, and Chiara Dall’Asta. 2016. "Characterization and Discrimination of Ancient Grains: A Metabolomics Approach" International Journal of Molecular Sciences 17, no. 8: 1217. https://doi.org/10.3390/ijms17081217
APA StyleRighetti, L., Rubert, J., Galaverna, G., Folloni, S., Ranieri, R., Stranska-Zachariasova, M., Hajslova, J., & Dall’Asta, C. (2016). Characterization and Discrimination of Ancient Grains: A Metabolomics Approach. International Journal of Molecular Sciences, 17(8), 1217. https://doi.org/10.3390/ijms17081217