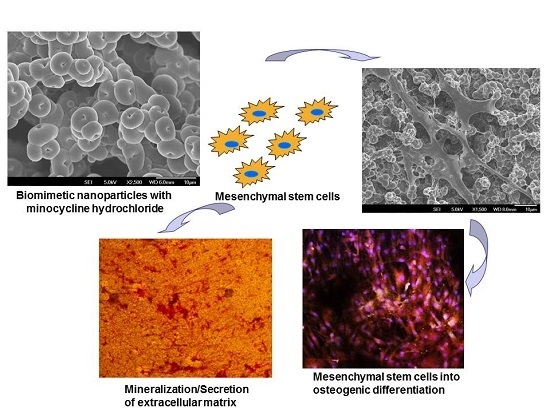
Minocycline Loaded Hybrid Composites Nanoparticles for Mesenchymal Stem Cells Differentiation into Osteogenesis
Abstract
:1. Introduction
2. Results and Discussion
2.1. Characterization of Nanoparticles
2.2. Particles Degradation and Minocycline Hydrochloride (MH) Release
2.3. Cell Morphology
2.4. Mineralization
2.5. Expression of Osteocalcin
3. Materials and Methods
3.1. Materials
3.2. Fabrication of Nanoparticle
3.3. Nanoparticles Characterization
3.4. Degradation and Drug Release
3.5. Cell Culture
3.6. Cell Proliferation
3.7. Cell Morphology
3.8. Alkaline Phosphatase Activity
3.9. Mineralization
3.10. Osteocalcin Expression
3.11. Statistical Analysis
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Hench, L.L.; Thompson, I. Twenty-first century challenges for biomaterials. J. R. Soc. Interface 2010, 7, S379–S391. [Google Scholar] [CrossRef] [PubMed]
- Schubert, T.; Xhema, D.; Vériter, S.; Schubert, M.; Behets, C.; Delloye, C. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials 2011, 32, 8880–8891. [Google Scholar] [CrossRef] [PubMed]
- Behr, B.; Tang, C.; Germann, G.; Longaker, M.T.; Quarto, N. Locally applied vascular endothelial growth factor A increases the osteogenic healing capacity of human adipose-derived stem cells by promoting osteogenic and endothelial differentiation. Stem Cells 2011, 29, 286–296. [Google Scholar] [CrossRef] [PubMed]
- Tien, Y.C.; Chih, T.T.; Lin, J.H.; Ju, C.P.; Lin, S.D. Augmentation of tendon-bone healing by the use of calcium-phosphate cement. J. Bone Jt. Surg. Br. 2004, 86, 1072–1076. [Google Scholar] [CrossRef]
- Huangfu, X.; Zhao, J. Tendon-bone healing enhancement using injectable tricalcium phosphate in a dog anterior cruciate ligament reconstruction model. Arthroscopy 2007, 23, 455–462. [Google Scholar] [CrossRef] [PubMed]
- Dvir, T.; Timko, B.P.; Kohane, D.S.; Langer, R. Nanotechnological strategies for engineering complex tissues. Nat. Nanotechnol. 2011, 6, 13–22. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.; Zhou, Y.; Fan, W. Hypoxia-mimicking mesoporous bioactive glass scaffolds with controllable cobalt ion release for bone tissue engineering. Biomaterials 2012, 33, 2076–2085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erices, A.; Conget, P.; Minguell, J.J. Mesenchymal progenitor cells in human umbilical cord blood. Br. J. Haematol. 2000, 109, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Franklin, D.M.; Leddy, H.A.; Robey, P.G.; Storms, R.W.; Gimble, J.M. Surface protein characterization of human adipose tissue-derived stromal cells. J. Cell. Physiol. 2001, 189, 54–63. [Google Scholar] [CrossRef] [PubMed]
- Marion, N.W.; Mao, J.J. Mesenchymal stem cells and tissue engineering. Methods Enzymol. 2006, 420, 339–361. [Google Scholar] [PubMed]
- Priscilla, C.; Aveline, J.; Bourseguin, I.; Rochefort, Y. Mesenchymal stem cells and acquisition of a bone phenotype: An ion channel overview. J. Dev. Biol. Tissue Eng. 2011, 3, 103–118. [Google Scholar]
- Lemmouchi, Y.; Schatch, E. Preparation and in vitro evaluation of biodegradable poly (ε-caprolactone-co-d,l lactide) (X–Y) devises containing tryparocidal drugs. J. Control. Release 1997, 45, 227–233. [Google Scholar] [CrossRef]
- Hardy, J.G.; Leal-Egaña, A.; Scheibel, T.R. Engineered spider silk protein-based composites for drug delivery. Macromol. Biosci. 2013, 13, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Numata, K.; Kaplan, D.L. Silk-based delivery systems of bioactive molecules. Adv. Drug Deliv. Rev. 2010, 62, 1497–1508. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fan, M.W.; Ma, Z.; Wu, C.; Fang, W.; Liu, G.; Xiao, Y. The effects of pore architecture in silk fibroin scaffolds on the growth and differentiation of mesenchymal stem cells expressing BMP7. Acta Biomater. 2010, 6, 3021–3028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meinel, L.; Fajardo, R.; Hofmann, S.; Langer, R.; Chen, J.; Snyder, B.; Vunjak-Novakovic, G.; Kaplan, D. Silk implants for the healing of critical size bone defects. Bone 2005, 37, 688–698. [Google Scholar] [CrossRef] [PubMed]
- Meinel, L.; Hofmann, S.; Betz, O.; Fajardo, R.; Merkle, H.P.; Langer, R.; Evans, C.H.; Vunjak-Novakovic, G.; Kaplan, D.L. Osteogenesis by human mesenchymal stem cells cultured on silk biomaterials: Comparison of adenovirus mediated gene transfer and protein delivery of BMP-2. Biomaterials 2006, 27, 4993–5002. [Google Scholar] [CrossRef] [PubMed]
- Young, D.S.; Krause, E.W.; Balik, C.M. Hyaluronic Acid-Based Nanofibers via Electrospinning. Master’s Thesis, North Carolina State University, Raleigh, NC, USA, 2006. [Google Scholar]
- Hosseini Ravandi, S.A.; Gandhimathi, C.; Valizadeh, M.; Ramakrishna, S. Application of electrospun natural biopolymer nanofibers. Curr. Nanosci. 2013, 9, 423–433. [Google Scholar] [CrossRef]
- Xiao, C.D.; Xiao, P.Z.; Jian, Z.; Hua-Qiong, C.; Jian, T.; Quan-Li, L. Minocycline-released hydroxyapatite–gelatin nanocomposite and its cytocompatibility in vitro. Biomed. Mater. 2011, 6, 5002. [Google Scholar] [CrossRef]
- Teixeria, A.I.; Nealey, P.P.F.; Murphy, C.J. Responses of human keratocytes to micro and nanostructured substrates. J. Biomed. Mater. Res. A 2004, 71, 369–376. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, P.; Rezaeian, I.; Ranaei-Siadat, S.O.; Jafari, S.H.; Supaphol, P. A review on wound dressings with an emphasis on electrospun nanofibrous polymeric bandages. Polym. Adv. Technol. 2010, 21, 77–95. [Google Scholar] [CrossRef]
- Maquet, V.; Boccaccini, A.R.; Pravata, L.; Notingher, I.; Jerome, R. Porous poly(ahydroxyacid)/Bioglass composite scaffolds for bone tissue engineering. I: Preparation and in vitro characterisation. Biomaterials 2004, 25, 4185–4194. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.Y.; Shi, M.; Goh, S.H.; Moochala, S.; Heller, J. POE/PLGA composite microspheres: Formation and in vitro behaviour of double walled microspheres. J. Control. Release 2003, 88, 201–213. [Google Scholar] [CrossRef]
- Loo, S.C.J.; Ooi, C.P.; Boey, Y.C.F. Radiation effects on poly(lactide-co-glycolide) (PLGA) and poly(l-lactide) (PLLA). Polym. Degrad. Stab. 2004, 83, 259–265. [Google Scholar] [CrossRef]
- Hurrell, S.; Cameron, R.E. Polyglycolide: Degradation and drug release. Part I: Changes morphology during degradation. J. Mater. Sci. Mater. Med. 2001, 12, 811–816. [Google Scholar] [CrossRef] [PubMed]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Venugopal, J.R.; Low, S.; Choon, A.T.; Kumar, A.B.; Ramakrishna, S. Nanobioengineered electrospun composite nanofibers and osteoblasts for bone regeneration. Artif. Organs 2008, 32, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Venugopal, J.; Rajeswari, R.; Shayanthi, M.; Bongso, A.; Giri Dev, V.R.; Deepika, G.; Choon, A.T.; Ramakrishna, S. Electrosprayed hydroxyapatite on polymer nanofibers to differentiate mesenchymal stem cells to osteogenesis. J. Biomater. Sci. Polym. Ed. 2013, 24, 170–184. [Google Scholar] [CrossRef] [PubMed]
- Anderson, H.C.; Sipe, J.B.; Hessle, L.; Dhanyamraju, R.; Atti, E.; Camacho, N.P. Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am. J. Pathol. 2004, 164, 841–847. [Google Scholar] [CrossRef]
- Liu, F.; Malaval, L.; Aubin, J.E. Global amplification polymerase chain reaction reveals novel transitional stages during osteoprogenitor differentiation. J. Cell Sci. 2003, 116, 1787–1796. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Chatvanichkul, K.I.; Barnes, G.L.; Latempa, T.J.; Grimes, C.A.; Desai, T.A. Osteogenic differentiation of marrow stromal cells cultured on nanoporous alumina surfaces. J. Biomed. Mater. Res. A 2007, 80, 955–964. [Google Scholar] [CrossRef] [PubMed]
- Popat, K.C.; Daniels, R.H.; Dubrow, R.S.; Hardev, V.; Desai, T.A. Nanostructured surfaces for bone biotemplating applications. J. Orthop. Res. 2006, 24, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Shim, J.H.; Kang, K.S.; Yeom, J.; Jung, H.S.; Kim, J.Y. Solid freeform fabrication of tissue-engineering scaffolds with a poly(lactic-co-glycolic acid) grafted hyaluronic acid conjugate encapsulating an intact bone morphogenetic protein–2/poly(ethylene glycol) complex. Adv. Funct. Mater. 2011, 21, 2906–2912. [Google Scholar] [CrossRef]
- Mizuno, M.; Kuboki, Y. Osteoblast-related gene expression of bone marrow cells during the osteoblastic differentiation induced by type I collagen. J. Biochem. 2001, 129, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Stein, G.S.; Lian, J.B.; van Wijnen, A.J.; Stein, J.L.; Montecino, M.; Javed, A. Runx2 control of organization, assembly and activity of the regulatory machinery for skeletal gene expression. Oncogene 2004, 23, 4315–4329. [Google Scholar] [CrossRef] [PubMed]
- Young, M.F.; Kerr, J.M.; Ibaraki, K.; Heegaard, A.M.; Robey, P.G. Structure, expression, and regulation of the major noncollagenous matrix proteins of bone. Clin. Orthop. Relat. Res. 1992, 281, 275–294. [Google Scholar] [CrossRef] [PubMed]













| Composite Particles | Particle Size (µm) |
|---|---|
| Polycaprolactone (PCL) | 3.2 ± 0.18 |
| Polycaprolactone/silk fibroin (PCL/SF) | 1.62 ± 0.59 |
| Polycaprolactone/silk fibroin/hyaluronic acid (PLC/SF/HA) | 0.9 ± 0.15 |
| Polycaprolactone/silk fibroin/hyaluronic acid/minocycline hydrochloride (PCL/SF/HA/MH) | 0.54 ± 0.12 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tham, A.Y.; Gandhimathi, C.; Praveena, J.; Venugopal, J.R.; Ramakrishna, S.; Kumar, S.D. Minocycline Loaded Hybrid Composites Nanoparticles for Mesenchymal Stem Cells Differentiation into Osteogenesis. Int. J. Mol. Sci. 2016, 17, 1222. https://doi.org/10.3390/ijms17081222
Tham AY, Gandhimathi C, Praveena J, Venugopal JR, Ramakrishna S, Kumar SD. Minocycline Loaded Hybrid Composites Nanoparticles for Mesenchymal Stem Cells Differentiation into Osteogenesis. International Journal of Molecular Sciences. 2016; 17(8):1222. https://doi.org/10.3390/ijms17081222
Chicago/Turabian StyleTham, Allister Yingwei, Chinnasamy Gandhimathi, Jayaraman Praveena, Jayarama Reddy Venugopal, Seeram Ramakrishna, and Srinivasan Dinesh Kumar. 2016. "Minocycline Loaded Hybrid Composites Nanoparticles for Mesenchymal Stem Cells Differentiation into Osteogenesis" International Journal of Molecular Sciences 17, no. 8: 1222. https://doi.org/10.3390/ijms17081222