Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury
Abstract
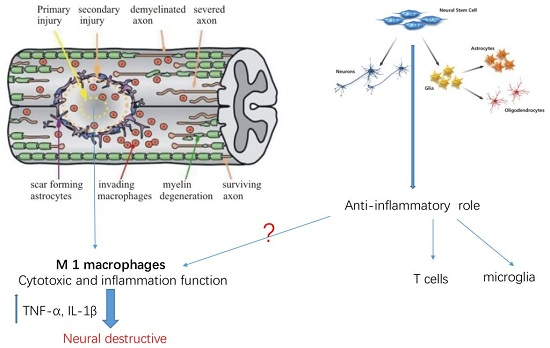
:1. Introduction
2. Result
2.1. Isolation and Characterization of NSCs
2.2. Survival, Migration and Distribution of Transplanted NSCs in Host Tissue
2.3. NSCs Transplantation Improved Functional Recovery after SCI
2.4. NSC Transplantation Reduced Neutrophils and Regulated the Activation of Macrophage
2.5. NSCs Attenuated mRNA Levels of Inflammatory Cytokines in the Spinal Cord Tissue
2.6. NSCs Inhibited BMDMs’ Activation and Reduced the Release of Inflammatory Cytokines by Macrophages in Vitro
3. Discussion
4. Materials and Methods
4.1. Experimental Animals
4.2. Reagents
4.3. Isolation and Identification of NSCs
4.4. Experimental Groups
4.5. Establishment of the SCI Animal Model
4.6. NSC Transplantation
4.7. Assessment of Locomotor Function
4.8. Tissue Preparation and Immunohistochemistry
4.9. RNA Isolation and Quantitative Real-Time PCR
4.10. Isolation of Macrophage
4.11. Enzyme-Linked Immunosorbent Assay
4.12. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Devivo, M.J. Epidemiology of traumatic spinal cord injury: Trends and future implications. Spinal Cord 2012, 50, 365–372. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Becker, D. Spinal cord injury: Promising interventions and realistic goals. Am. J. Phys. Med. Rehabil. 2003, 82, 38–49. [Google Scholar] [CrossRef] [PubMed]
- McDonald, J.W.; Sadowsky, C. Spinal-cord injury. Lancet 2002, 359, 417–425. [Google Scholar] [CrossRef]
- Rhoney, D.H.; Luer, M.S.; Hughes, M.; Hatton, J. New pharmacologic approaches to acute spinal cord injury. Pharmacotherapy 1996, 16, 382–392. [Google Scholar] [PubMed]
- Hausmann, O.N. Post-traumatic inflammation following spinal cord injury. Spinal Cord 2003, 41, 369–378. [Google Scholar] [CrossRef] [PubMed]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Ourednik, J.; Ourednik, V.; Lynch, W.P.; Schachner, M.; Snyder, E.Y. Neural stem cells display an inherent mechanism for rescuing dysfunctional neurons. Nat. Biotechnol. 2002, 20, 1103–1110. [Google Scholar] [CrossRef] [PubMed]
- Okano, H. Stem cell biology of the central nervous system. J. Neurosci. Res. 2002, 69, 698–707. [Google Scholar] [CrossRef]
- Ming, G.L.; Song, H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011, 70, 687–702. [Google Scholar] [CrossRef] [PubMed]
- Parr, A.M.; Kulbatski, I.; Tator, C.H. Transplantation of adult rat spinal cord stem/progenitor cells for spinal cord injury. J. Neurotrauma 2007, 24, 835–845. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S.; Yasuda, A.; Iwai, H.; Takano, M.; Kobayashi, Y.; Nori, S.; Tsuji, O.; Fujiyoshi, K.; Ebise, H.; Toyama, Y.; et al. Time-dependent changes in the microenvironment of injured spinal cord affects the therapeutic potential of neural stem cell transplantation for spinal cord injury. Mol. Brain 2013, 6, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Karimi-Abdolrezaee, S.; Eftekharpour, E.; Wang, J.; Morshead, C.M.; Fehlings, M.G. Delayed transplantation of adult neural precursor cells promotes remyelination and functional neurological recovery after spinal cord injury. J. Neurosci. 2006, 26, 3377–3389. [Google Scholar] [CrossRef] [PubMed]
- Cusimano, M.; Biziato, D.; Brambilla, E.; Donega, M.; Alfaro-Cervello, C.; Snider, S.; Salani, G.; Pucci, F.; Comi, G.; Garcia-Verdugo, J.M.; et al. Transplanted neural stem/precursor cells instruct phagocytes and reduce secondary tissue damage in the injured spinal cord. Brain 2012, 135, 447–460. [Google Scholar] [CrossRef] [PubMed]
- Einstein, O.; Ben-Hur, T. The changing face of neural stem cell therapy in neurologic diseases. Arch. Neurol. 2008, 65, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Einstein, O.; Karussis, D.; Grigoriadis, N.; Mizrachi-Kol, R.; Reinhartz, E.; Abramsky, O.; Ben-Hur, T. Intraventricular transplantation of neural precursor cell spheres attenuates acute experimental allergic encephalomyelitis. Mol. Cell. Neurosci. 2003, 24, 1074–1082. [Google Scholar] [CrossRef] [PubMed]
- Pluchino, S.; Zanotti, L.; Rossi, B.; Brambilla, E.; Ottoboni, L.; Salani, G.; Martinello, M.; Cattalini, A.; Bergami, A.; Furlan, R.; et al. Neurosphere-derived multipotent precursors promote neuroprotection by an immunomodulatory mechanism. Nature 2005, 436, 266–271. [Google Scholar] [CrossRef] [PubMed]
- Aharonowiz, M.; Einstein, O.; Fainstein, N.; Lassmann, H.; Reubinoff, B.; Ben-Hur, T. Neuroprotective effect of transplanted human embryonic stem cell-derived neural precursors in an animal model of multiple sclerosis. PLoS ONE 2008, 3, e3145. [Google Scholar] [CrossRef] [PubMed]
- Einstein, O.; Fainstein, N.; Vaknin, I.; Mizrachi-Kol, R.; Reihartz, E.; Grigoriadis, N.; Lavon, I.; Baniyash, M.; Lassmann, H.; Ben-Hur, T. Neural precursors attenuate autoimmune encephalomyelitis by peripheral immunosuppression. Ann. Neurol. 2007, 61, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Mosher, K.I.; Andres, R.H.; Fukuhara, T.; Bieri, G.; Hasegawa-Moriyama, M.; He, Y.; Guzman, R.; Wyss-Coray, T. Neural progenitor cells regulate microglia functions and activity. Nat. Neurosci. 2012, 15, 1485–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, J.; Hjorth, E.; Zhu, M.; Calzarossa, C.; Samuelsson, E.B.; Schultzberg, M.; Akesson, E. Interplay between human microglia and neural stem/progenitor cells in an allogeneic co-culture model. J. Cell. Mol. Med. 2013, 17, 1434–1443. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Cao, K.; Sun, X.; Chen, Y.; Duan, Z.; Sun, L.; Guo, L.; Bai, P.; Sun, D.; Fan, J.; et al. Macrophages in spinal cord injury: Phenotypic and functional change from exposure to myelin debris. Glia 2015, 63, 635–651. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, H.; Uchida, K.; Guerrero, A.R.; Watanabe, S.; Sugita, D.; Takeura, N.; Yoshida, A.; Long, G.; Wright, K.T.; Johnson, W.E.; et al. Transplantation of mesenchymal stem cells promotes an alternative pathway of macrophage activation and functional recovery after spinal cord injury. J. Neurotrauma 2012, 29, 1614–1625. [Google Scholar] [CrossRef] [PubMed]
- Ziebell, J.M.; Morganti-Kossmann, M.C. Involvement of pro- and anti-inflammatory cytokines and chemokines in the pathophysiology of traumatic brain injury. Neurotherapeutics 2010, 7, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Lau, L.T.; Yu, A.C. Astrocytes produce and release interleukin-1, interleukin-6, tumor necrosis factor α and interferon-γ following traumatic and metabolic injury. J. Neurotrauma 2001, 18, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Pineau, I.; Lacroix, S. Proinflammatory cytokine synthesis in the injured mouse spinal cord: Multiphasic expression pattern and identification of the cell types involved. J. Comp. Neurol. 2007, 500, 267–285. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Blumbergs, P.C.; Jones, N.R.; Manavis, J.; Sarvestani, G.T.; Ghabriel, M.N. Early expression and cellular localization of proinflammatory cytokines interleukin-1β, interleukin-6, and tumor necrosis factor-α in human traumatic spinal cord injury. Spine 2004, 29, 966–971. [Google Scholar] [CrossRef] [PubMed]
- Gregersen, R.; Lambertsen, K.; Finsen, B. Microglia and macrophages are the major source of tumor necrosis factor in permanent middle cerebral artery occlusion in mice. J. Cereb. Blood Flow Metab. 2000, 20, 53–65. [Google Scholar] [CrossRef] [PubMed]
- Esposito, E.; Cuzzocrea, S. Anti-TNF therapy in the injured spinal cord. Trends Pharmacol. Sci. 2011, 32, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Boato, F.; Rosenberger, K.; Nelissen, S.; Geboes, L.; Peters, E.M.; Nitsch, R.; Hendrix, S. Absence of IL-1β positively affects neurological outcome, lesion development and axonal plasticity after spinal cord injury. J. Neuroinflamm. 2013, 10, 6–17. [Google Scholar] [CrossRef] [PubMed]
- Popovich, P.G.; Hickey, W.F. Bone marrow chimeric rats reveal the unique distribution of resident and recruited macrophages in the contused rat spinal cord. J. Neuropathol. Exp. Neurol. 2001, 60, 676–685. [Google Scholar] [CrossRef] [PubMed]
- Hawthorne, A.L.; Popovich, P.G. Emerging concepts in myeloid cell biology after spinal cord injury. Neurotherapeutics 2011, 8, 252261. [Google Scholar] [CrossRef] [PubMed]
- Shechter, R.; Schwartz, M. Harnessing monocyte-derived macrophages to control central nervous system pathologies: No longer “if” but “how”. J. Pathol. 2013, 229, 332–346. [Google Scholar] [CrossRef] [PubMed]
- Kigerl, K.A.; Gensel, J.C.; Ankeny, D.P.; Alexander, J.K.; Donnelly, D.J.; Popovich, P.G. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009, 29, 13435–13444. [Google Scholar] [CrossRef] [PubMed]
- Horn, K.P.; Busch, S.A.; Hawthorne, A.L.; van Rooijen, N.; Silver, J. Another barrier to regeneration in the CNS: Activated macrophages induce extensive retraction of dystrophic axons through direct physical interactions. J. Neurosci. 2008, 28, 9330–9341. [Google Scholar] [CrossRef] [PubMed]
- Andrade, M.R.; Amaral, E.P.; Ribeiro, S.C.; Almeida, F.M.; Peres, T.V.; Lanes, V.; D’Imperio-Lima, M.R.; Lasunskaia, E.B. Pathogenic mycobacterium bovis strains differ in their ability to modulate the proinflammatory activation phenotype of macrophages. BMC Microbiol. 2012, 12, 166–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guerrero, A.R.; Uchida, K.; Nakajima, H.; Watanabe, S.; Nakamura, M.; Johnson, W.E.; Baba, H. Blockade of interleukin-6 signaling inhibits the classic pathway and promotes an alternative pathway of macrophage activation after spinal cord injury in mice. J. Neuroinflamm. 2012, 9, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.T.; Chu, K.; Jung, K.H.; Kim, S.J.; Kim, D.H.; Kang, K.M.; Hong, N.H.; Kim, J.H.; Ban, J.J.; Park, H.K.; et al. Anti-inflammatory mechanism of intravascular neural stem cell transplantation in haemorrhagic stroke. Brain 2008, 131, 616–629. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Sun, W.; Han, D.W.; Moon, H.J.; Lee, J. iNSC suppress macrophage-induced inflammation by repressing COX-2. In Vitro Cell. Dev. Biol. Anim. 2015, 51, 157–164. [Google Scholar] [CrossRef] [PubMed]
- Forstreuter, F.; Lucius, R.; Mentlein, R. Vascular endothelial growth factor induces chemotaxis and proliferation of microglial cells. J. Neuroimmunol. 2002, 132, 93–98. [Google Scholar] [CrossRef]
- Busch, S.A.; Hamilton, J.A.; Horn, K.P.; Cuascut, F.X.; Cutrone, R.; Lehman, N.; Deans, R.J.; Ting, A.E.; Mays, R.W.; Silver, J. Multipotent adult progenitor cells prevent macrophage-mediated axonal dieback and promote regrowth after spinal cord injury. J. Neurosci. 2011, 31, 944–953. [Google Scholar] [CrossRef] [PubMed]
- Baglio, S.R.; Rooijers, K.; Koppers-Lalic, D.; Verweij, F.J.; Perez Lanzon, M.; Zini, N.; Naaijkens, B.; Perut, F.; Niessen, H.W.; Baldini, N.; et al. Human bone marrow- and adipose-mesenchymal stem cells secrete exosomes enriched in distinctive miRNA and tRNA species. Stem Cell Res. Ther. 2015, 6, 127–147. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Young, W. Managing inflammation after spinal cord injury through manipulation of macrophage function. Neural Plast. 2013, 2013, 945034–945043. [Google Scholar] [CrossRef] [PubMed]
- Huie, J.R.; Baumbauer, K.M.; Lee, K.H.; Bresnahan, J.C.; Beattie, M.S.; Ferguson, A.R.; Grau, J.W. Glial tumor necrosis factor α (TNFα) generates metaplastic inhibition of spinal learning. PLoS ONE 2012, 7, e39751. [Google Scholar] [CrossRef] [PubMed]
- Kwon, B.K.; Okon, E.; Hillyer, J.; Mann, C.; Baptiste, D.; Weaver, L.C.; Fehlings, M.G.; Tetzlaff, W. A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma 2011, 28, 1545–1588. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Wu, C.; Xiong, Q.; Zhou, L.; Tian, Y. Anti-inflammatory mechanism of bone marrow mesenchymal stem cell transplantation in rat model of spinal cord injury. Cell Biochem. Biophys. 2015, 71, 1341–1347. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wang, X.; Guo, L.; Wu, M.; Duan, Z.; Lv, J.; Tai, W.; Renganathan, H.; Didier, R.; Li, J.; et al. Embryonic stem cells promoting macrophage survival and function are crucial for teratoma development. Front. Immunol. 2014, 5, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, K.; Leelahavanichkul, A.; Yuen, P.S.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone marrow stromal cells attenuate sepsis via prostaglandin E(2)-dependent reprogramming of host macrophages to increase their interleukin-10 production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Hematti, P. Mesenchymal stem cell-educated macrophages: A novel type of alternatively activated macrophages. Exp. Hematol. 2009, 37, 1445–1453. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.; Rolfe, A.J.; Wang, X.; Tai, W.; Cheng, Z.; Cao, K.; Chen, X.; Xu, Y.; Sun, D.; Li, J.; et al. Rescuing macrophage normal function in spinal cord injury with embryonic stem cell conditioned media. Mol. Brain 2016, 9, 48–62. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, B.A.; Tetzlaff, W.; Weiss, S. A multipotent EGF-responsive striatal embryonic progenitor cell produces neurons and astrocytes. J. Neurosci. 1992, 12, 4565–4574. [Google Scholar] [PubMed]
- Young, W. Spinal cord contusion models. Prog. Brain Res. 2002, 137, 231–255. [Google Scholar] [PubMed]
- Salewski, R.P.; Mitchell, R.A.; Shen, C.; Fehlings, M.G. Transplantation of neural stem cells clonally derived from embryonic stem cells promotes recovery after murine spinal cord injury. Stem Cells Dev. 2015, 24, 36–50. [Google Scholar] [CrossRef] [PubMed]
- Basso, D.M.; Fisher, L.C.; Anderson, A.J.; Jakeman, L.B.; McTigue, D.M.; Popovich, P.G. Basso mouse scale for locomotion detects differences in recovery after spinal cord injury in five common mouse strains. J. Neurotrauma 2006, 23, 635–659. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.F.; Cen, J.S.; Zhong, Q.; Chen, L.; Wang, J.; Deng, D.Y.; Wan, Y. The promotion of functional recovery and nerve regeneration after spinal cord injury by lentiviral vectors encoding lingo-1 shRNA delivered by pluronic F-127. Biomaterials 2013, 34, 1686–1700. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Chen, T.; Leng, L.; Fan, J.; Cao, K.; Duan, Z.; Zhang, X.; Shao, C.; Wu, M.; Tadmori, I.; et al. MIF produced by bone marrow-derived macrophages contributes to teratoma progression after embryonic stem cell transplantation. Cancer Res. 2012, 72, 2867–2878. [Google Scholar] [CrossRef] [PubMed]








| Antigen | Catalog Number | Dilution | Source |
|---|---|---|---|
| GFAP | Ab53554 | 1:600 | Abcam |
| iNOS | 610329 | 1:600 | BD Biosciences |
| Nestin | Ab24692 | 1:300 | Abcam |
| Neutrophil (7/4) | AB53457 | 1:400 | Abcam |
| O4 | MAB1326 | 1:100 | R & D Systems |
| Tuj1 | 04-1049 | 1:400 | Millipore |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, Z.; Zhu, W.; Cao, K.; Wu, F.; Li, J.; Wang, G.; Li, H.; Lu, M.; Ren, Y.; He, X. Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury. Int. J. Mol. Sci. 2016, 17, 1380. https://doi.org/10.3390/ijms17091380
Cheng Z, Zhu W, Cao K, Wu F, Li J, Wang G, Li H, Lu M, Ren Y, He X. Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury. International Journal of Molecular Sciences. 2016; 17(9):1380. https://doi.org/10.3390/ijms17091380
Chicago/Turabian StyleCheng, Zhijian, Wen Zhu, Kai Cao, Fei Wu, Jin Li, Guoyu Wang, Haopen Li, Ming Lu, Yi Ren, and Xijing He. 2016. "Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury" International Journal of Molecular Sciences 17, no. 9: 1380. https://doi.org/10.3390/ijms17091380
APA StyleCheng, Z., Zhu, W., Cao, K., Wu, F., Li, J., Wang, G., Li, H., Lu, M., Ren, Y., & He, X. (2016). Anti-Inflammatory Mechanism of Neural Stem Cell Transplantation in Spinal Cord Injury. International Journal of Molecular Sciences, 17(9), 1380. https://doi.org/10.3390/ijms17091380