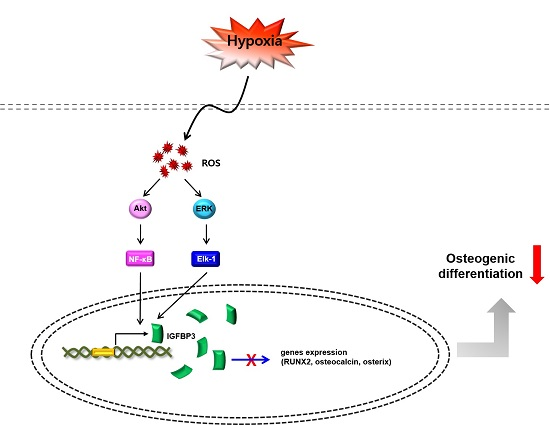
Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of Mesenchymal Stem Cells via IGFBP3 Up-Regulation
Abstract
:1. Introduction
2. Results and Discussion
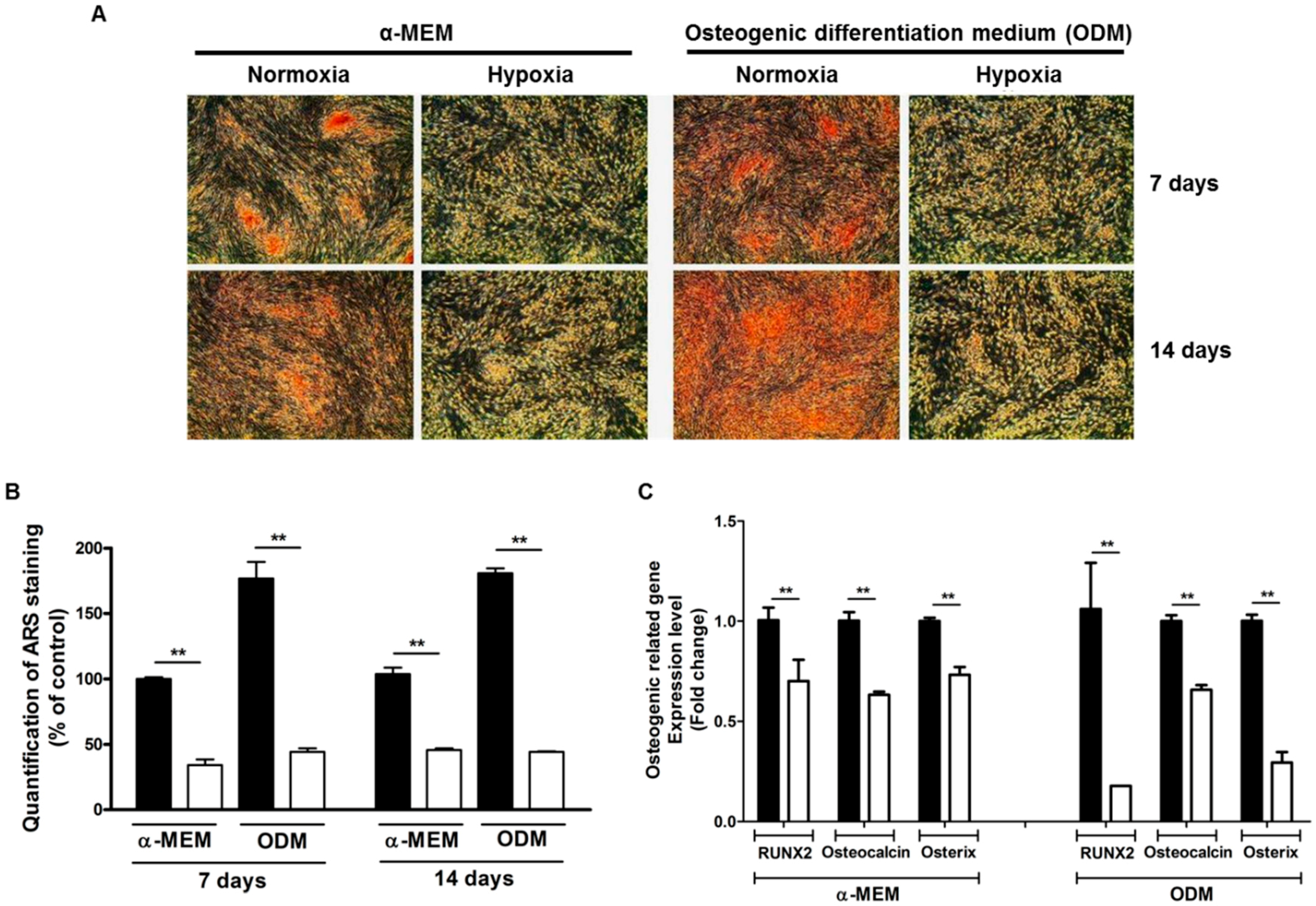
2.1. Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of ASCs
2.2. Hypoxia Induces IGFBP Expression
2.3. Recombinant IGFBP3–6 Does Not Suppress the Osteogenic Differentiation of ASCs
2.4. Role of Intracellular IGFBP3 in Osteogenic Differentiation
2.5. ROS Up-Regulates IGFBP3 Expression
2.6. Hypoxia Suppresses the Osteogenic Differentiation of Clonal BM-MSCs via IGFBP3
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Osteogenic Differentiation
4.3. Spontaneous Mineralization
4.4. Alizarin Red S Staining
4.5. Membrane-Based Human Growth Factor Assay
4.6. siRNA Transfection
4.7. IGFBP3 Overexpression
4.8. Immunostaining of IGFBP3
4.9. Immunoprecipitation Assay
4.10. RNA Isolation and Quantitative Real-Time PCR
4.11. Cell Proliferation
4.12. Animal Study
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Chung, H.M.; Won, C.H.; Sung, J.H.; Patel, B. Responses of adipose-derived stem cells during hypoxia: Enhanced skin-regenerative potential. Expert Opin. Biol. Ther. 2009, 9, 1499–1508. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Kim, S.M.; Sung, J.H. Cellular and molecular stimulation of adipose-derived stem cells under hypoxia. Cell Biol. Int. 2014, 38, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.H.; Park, S.G.; Choi, J.S.; Xia, Y.; Sung, J.H. The pivotal role of reactive oxygen species generation in the hypoxia-induced stimulation of adipose-derived stem cells. Stem Cells Dev. 2011, 20, 1753–1761. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Song, S.Y.; Park, S.G.; Song, S.U.; Xia, Y.; Sung, J.H. Primary involvement of NADPH oxidase 4 in hypoxia-induced generation of reactive oxygen species in adipose-derived stem cells. Stem Cells Dev. 2012, 21, 2212–2221. [Google Scholar] [CrossRef] [PubMed]
- Jee, M.K.; Kim, J.H.; Han, Y.M.; Jung, S.J.; Kang, K.S.; Kim, D.W.; Kang, S.K. DHP-derivative and low oxygen tension effectively induces human adipose stromal cell reprogramming. PLoS ONE 2010, 5, e9026. [Google Scholar] [CrossRef] [PubMed]
- Lee, E.Y.; Xia, Y.; Kim, W.S.; Kim, M.H.; Kim, T.H.; Kim, K.J.; Park, B.S.; Sung, J.H. Hypoxia-enhanced wound-healing function of adipose-derived stem cells: Increase in stem cell proliferation and up-regulation of VEGF and bFGF. Wound Repair Regen. 2009, 17, 540–547. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, W.S.; Sung, Y.K.; Kwack, M.H.; Song, S.Y.; Choi, J.S.; Park, S.G.; Yi, T.; Lee, H.J.; Kim, D.D.; et al. The molecular mechanism underlying the proliferating and preconditioning effect of vitamin C on adipose-derived stem cells. Stem Cells Dev. 2014, 23, 1364–1376. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.G.; Kim, W.K.; Song, S.U.; Sung, J.H. Functional regulation of adipose-derived stem cells by PDGF-D. Stem Cells 2015, 33, 542–556. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Park, S.G.; Song, S.Y.; Kim, J.K.; Sung, J.H. Reactive oxygen species-responsive miR-210 regulates proliferation and migration of adipose-derived stem cells via PTPN2. Cell Death Dis. 2013, 4, e588. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Kim, W.S.; Choi, J.S.; Kim, H.K.; Won, J.H.; Ohkubo, F.; Fukuoka, H. Hair growth stimulated by conditioned medium of adipose-derived stem cells is enhanced by hypoxia: Evidence of increased growth factor secretion. BioMed Res. 2010, 31, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Kim, S.H.; Song, S.Y.; Kim, W.S.; Song, S.U.; Yi, T.; Jeon, M.S.; Chung, H.M.; Xia, Y.; Sung, J.H. Hypoxia induces adipocyte differentiation of adipose-derived stem cells by triggering reactive oxygen species generation. Cell Biol. Int. 2014, 38, 32–40. [Google Scholar] [CrossRef] [PubMed]
- Sahai, S.; Williams, A.; Skiles, M.L.; Blanchette, J.O. Osteogenic differentiation of adipose-derived stem cells is hypoxia-inducible factor-1 independent. Tissue Eng. Part A 2013, 19, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Valorani, M.G.; Montelatici, E.; Germani, A.; Biddle, A.; D’Alessandro, D.; Strollo, R.; Patrizi, M.P.; Lazzari, L.; Nye, E.; Otto, W.R.; et al. Pre-culturing human adipose tissue mesenchymal stem cells under hypoxia increases their adipogenic and osteogenic differentiation potentials. Cell Prolif. 2012, 45, 225–238. [Google Scholar] [CrossRef]
- Weijers, E.M.; Van Den Broek, L.J.; Waaijman, T.; Van Hinsbergh, V.W.; Gibbs, S.; Koolwijk, P. The influence of hypoxia and fibrinogen variants on the expansion and differentiation of adipose tissue-derived mesenchymal stem cells. Tissue Eng. Part A 2011, 17, 2675–2685. [Google Scholar] [CrossRef] [PubMed]
- Khan, W.S.; Adesida, A.B.; Hardingham, T.E. Hypoxic conditions increase hypoxia-inducible transcription factor 2α and enhance chondrogenesis in stem cells from the infrapatellar fat pad of osteoarthritis patients. Arthritis Res. Ther. 2007, 9, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Merceron, C.; Vinatier, C.; Portron, S.; Masson, M.; Amiaud, J.; Guigand, L.; Cherel, Y.; Weiss, P.; Guicheux, J. Differential effects of hypoxia on osteochondrogenic potential of human adipose-derived stem cells. Am. J. Physiol. Cell Physiol. 2010, 298, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Hsu, S.H.; Chen, C.T.; Wei, Y.H. Inhibitory effects of hypoxia on metabolic switch and osteogenic differentiation of human mesenchymal stem cells. Stem Cells 2013, 31, 2779–2788. [Google Scholar] [CrossRef] [PubMed]
- Meretoja, V.V.; Dahlin, R.L.; Wright, S.; Kasper, F.K.; Mikos, A.G. The effect of hypoxia on the chondrogenic differentiation of co-cultured articular chondrocytes and mesenchymal stem cells in scaffolds. Biomaterials 2013, 34, 4266–4273. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Genetos, D.C.; Yellowley, C.E.; Leach, J.K. Oxygen tension differentially influences osteogenic differentiation of human adipose stem cells in 2D and 3D cultures. J. Cell. Biochem. 2010, 110, 87–96. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.C.; Zhu, H.M.; Cai, J.Q.; Huang, Y.Z.; Xu, J.; Zhou, Y.; Chen, X.H.; Li, X.Q.; Yang, Z.M.; Deng, L. Hypoxia inhibits the spontaneous calcification of bone marrow-derived mesenchymal stem cells. J. Cell. Biochem. 2012, 113, 1407–1415. [Google Scholar] [CrossRef] [PubMed]
- Tseng, P.C.; Young, T.H.; Wang, T.M.; Peng, H.W.; Hou, S.M.; Yen, M.L. Spontaneous osteogenesis of MSCs cultured on 3D microcarriers through alteration of cytoskeletal tension. Biomaterials 2012, 33, 556–564. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.S.; Sung, J.H. Hypoxic Culturing Enhances the Wound-Healing Potential of Adipose-Derived Stem Cells. Adv. Wound Care 2012, 1, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Song, S.Y.; Chung, H.M.; Sung, J.H. The pivotal role of VEGF in adipose-derived-stem-cell-mediated regeneration. Expert Opin. Biol. Ther. 2010, 10, 1529–1537. [Google Scholar] [CrossRef] [PubMed]
- Jung, K.H.; Song, S.U.; Yi, T.; Jeon, M.S.; Hong, S.W.; Zheng, H.M.; Lee, H.S.; Choi, M.J.; Lee, D.H.; Hong, S.S. Human bone marrow-derived clonal mesenchymal stem cells inhibit inflammation and reduce acute pancreatitis in rats. Gastroenterology 2011, 140, 998–1008. [Google Scholar] [CrossRef] [PubMed]
- Song, S.U.; Kim, C.S.; Yoon, S.P.; Kim, S.K.; Lee, M.H.; Kang, J.S.; Choi, G.S.; Moon, S.H.; Choi, M.S.; Cho, Y.K.; et al. Variations of clonal marrow stem cell lines established from human bone marrow in surface epitopes, differentiation potential, gene expression, and cytokine secretion. Stem Cells Dev. 2008, 17, 451–461. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Li, J.; Wang, Y.; Lei, L.; Jiang, C.; An, S.; Zhan, Y.; Cheng, Q.; Zhao, Z.; Wang, J.; et al. Effects of hypoxia on osteogenic differentiation of rat bone marrow mesenchymal stem cells. Mol. Cell. Biochem. 2012, 362, 25–33. [Google Scholar] [CrossRef] [PubMed]
- Ismail, S.; Sturrock, A.; Wu, P.; Cahill, B.; Norman, K.; Huecksteadt, T.; Sanders, K.; Kennedy, T.; Hoidal, J. NOX4 mediates hypoxia-induced proliferation of human pulmonary artery smooth muscle cells: The role of autocrine production of transforming growth factor-β1 and insulin-like growth factor binding protein 3. Am. J. Physiol. Lung Cell. Mol. Physiol. 2009, 396, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Jiang, W.; Huang, J.; He, B.C.; Zuo, G.W.; Zhang, W.; Luo, Q.; Shi, Q.; Zhang, B.Q.; Wagner, E.R.; et al. Insulin-like growth factor 2 (IGF-2) potentiates BMP-9-induced osteogenic differentiation and bone formation. J. Bone Miner. Res. 2010, 25, 2447–2459. [Google Scholar] [CrossRef] [PubMed]
- Al-Kharobi, H.; El-Gendy, R.; Devine, D.A.; Beattie, J. The role of the insulin-like growth factor (IGF) axis in osteogenic and odontogenic differentiation. Cell. Mol. Life Sci. 2014, 71, 1469–1476. [Google Scholar] [CrossRef] [PubMed]
- Siddals, K.W.; Allen, J.; Sinha, S.; Canfield, A.E.; Kalra, P.A.; Gibson, J.M. Apposite insulin-like growth factor (IGF) receptor glycosylation is critical to the maintenance of vascular smooth muscle phenotype in the presence of factors promoting osteogenic differentiation and mineralization. J. Biol. Chem. 2011, 286, 16623–16630. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. IGFBP special issue—Introduction. J. Cell Commun. Signal. 2015, 9, 109. [Google Scholar] [CrossRef] [PubMed]
- Baxter, R.C. Insulin-like growth factor binding protein 3 (IGFBP3): Novel ligands mediate unexpected functions. J. Cell Commun. Signal. 2013, 7, 179–189. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.S.; Schedlich, L.J.; Twigg, S.M.; Baxter, R.C. Inhibition of adipocyte differentiation by insulin-like growth factor-binding protein-3. Am. J. Physiol. Endocrinol. Metab. 2009, 296, 654–663. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jin, D.; Fu, S.; Mei, G.; Zhou, J.; Lei, L.; Yu, B.; Wang, G. Insulin-like growth factor binding protein 3 modulates osteoblast differentiation via interaction with vitamin D receptor. Biochem. Biophys. Res. Commun. 2013, 436, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Ikezoe, T.; Tanosaki, S.; Krug, U.; Liu, B.; Cohen, P.; Taguchi, H.; Koeffler, H.P. Insulin-like growth factor binding protein 3 antagonizes the effects of retinoids in myeloid leukemia cells. Blood 2004, 104, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Schedlich, L.J.; O’Han, M.K.; Leong, G.M.; Baxter, R.C. Insulin-like growth factor binding protein 3 prevents retinoid receptor heterodimerization: Implications for retinoic acid-sensitivity in human breast cancer cells. Biochem. Biophys. Res. Commun. 2004, 314, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Natsuizaka, M.; Kinugasa, H.; Kagawa, S.; Whelan, K.A.; Naganuma, S.; Subramanian, H.; Chang, S.; Nakagawa, K.J.; Rustgi, N.L.; Kita, Y.; et al. IGFBP3 promotes esophageal cancer growth by suppressing oxidative stress in hypoxic tumor microenvironment. Am. J. Cancer Res. 2014, 4, 29–41. [Google Scholar] [PubMed]
- Baxter, R.C. IGF binding proteins in cancer: Mechanistic and clinical insights. Nat. Rev. Cancer 2014, 14, 329–341. [Google Scholar] [CrossRef] [PubMed]
- Probst-Hensch, N.M.; Steiner, J.H.; Schraml, P.; Varga, Z.; Zurrer-Hardi, U.; Storz, M.; Korol, D.; Fehr, M.K.; Fink, D.; Pestalozzi, B.C.; et al. IGFBP2 and IGFBP3 protein expressions in human breast cancer: Association with hormonal factors and obesity. Clin. Cancer Res. 2010, 16, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Martino, D.E.; Wild, C.P.; Rotimi, O.; Darnton, J.S.; Olliver, R.J.; Hardie, L.J. IGFBP3 and IGFBP10 (CYR61) up-regulation during the development of Barrett’s oesophagus and associated oesophageal adenocarcinoma: Potential biomarkers of disease risk. Biomarkers 2006, 16, 547–561. [Google Scholar] [CrossRef] [PubMed]
- Pon, C.K.; Firth, S.M.; Baxter, R.C. Involvement of insulin-like growth factor binding protein 3 in peroxisome proliferator-activated receptor γ-mediated inhibition of breast cancer cell growth. Mol. Cell. Endocrinol. 2015, 399, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.W.; Ma, L.; Yan, X.; Liu, B.; Zhang, X.K.; Cohen, P. Rapid apoptosis induction by IGFBP3 involves an insulin-like growth factor-independent nucleomitochondrial translocation of RXRα/Nur77. J. Biol. Chem. 2005, 280, 16942–16948. [Google Scholar] [CrossRef] [PubMed]
- Cobb, L.J.; Liu, B.; Lee, K.W.; Cohen, P. Phosphorylation by DNA-dependent protein kinase is critical for apoptosis induction by insulin-like growth factor binding protein 3. Cancer Res. 2006, 66, 10878–10884. [Google Scholar] [CrossRef] [PubMed]





| Gene | Ct Value | |
|---|---|---|
| 1 | IGF1 | not detected |
| 2 | IGF2 | 31.55 (±0.35) |
| 3 | IGF1R | 30.14 (±0.19) |
| 4 | IGF2R | not detected |
| 5 | IGFBP1 | not detected |
| 6 | IGFBP2 | not detected |
| 7 | IGFBP3 | 21.92 (±0.12) |
| 8 | IGFBP4 | 25.38 (±0.04) |
| 9 | IGFBP5 | 25.89 (±0.04) |
| 10 | IGFBP6 | 21.52 (±0.09) |
| 11 | IGFBP7 | 19.91 (±0.04) |
| 12 | GAPDH | 19.61 (±0.08) |
| 13 | 18S | 24.74 (±0.08) |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, J.H.; Yoon, S.M.; Song, S.U.; Park, S.G.; Kim, W.-S.; Park, I.G.; Lee, J.; Sung, J.-H. Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of Mesenchymal Stem Cells via IGFBP3 Up-Regulation. Int. J. Mol. Sci. 2016, 17, 1389. https://doi.org/10.3390/ijms17091389
Kim JH, Yoon SM, Song SU, Park SG, Kim W-S, Park IG, Lee J, Sung J-H. Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of Mesenchymal Stem Cells via IGFBP3 Up-Regulation. International Journal of Molecular Sciences. 2016; 17(9):1389. https://doi.org/10.3390/ijms17091389
Chicago/Turabian StyleKim, Ji Hye, Sei Mee Yoon, Sun U. Song, Sang Gyu Park, Won-Serk Kim, In Guk Park, Jinu Lee, and Jong-Hyuk Sung. 2016. "Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of Mesenchymal Stem Cells via IGFBP3 Up-Regulation" International Journal of Molecular Sciences 17, no. 9: 1389. https://doi.org/10.3390/ijms17091389
APA StyleKim, J. H., Yoon, S. M., Song, S. U., Park, S. G., Kim, W.-S., Park, I. G., Lee, J., & Sung, J.-H. (2016). Hypoxia Suppresses Spontaneous Mineralization and Osteogenic Differentiation of Mesenchymal Stem Cells via IGFBP3 Up-Regulation. International Journal of Molecular Sciences, 17(9), 1389. https://doi.org/10.3390/ijms17091389