Efficacy of Polyvalent Human Immunoglobulins in an Animal Model of Neuromyelitis Optica Evoked by Intrathecal Anti-Aquaporin 4 Antibodies
Abstract
:1. Introduction
2. Results
2.1. Systemic Treatment with Pooled Human Immunoglobulins (IVIg) Reduces Disease Progression in the NMO Immunoglobulin G (NMO-IgG) Passive-Transfer Model
2.2. Therapeutic Potential of Systemic IVIg on Disease Progression
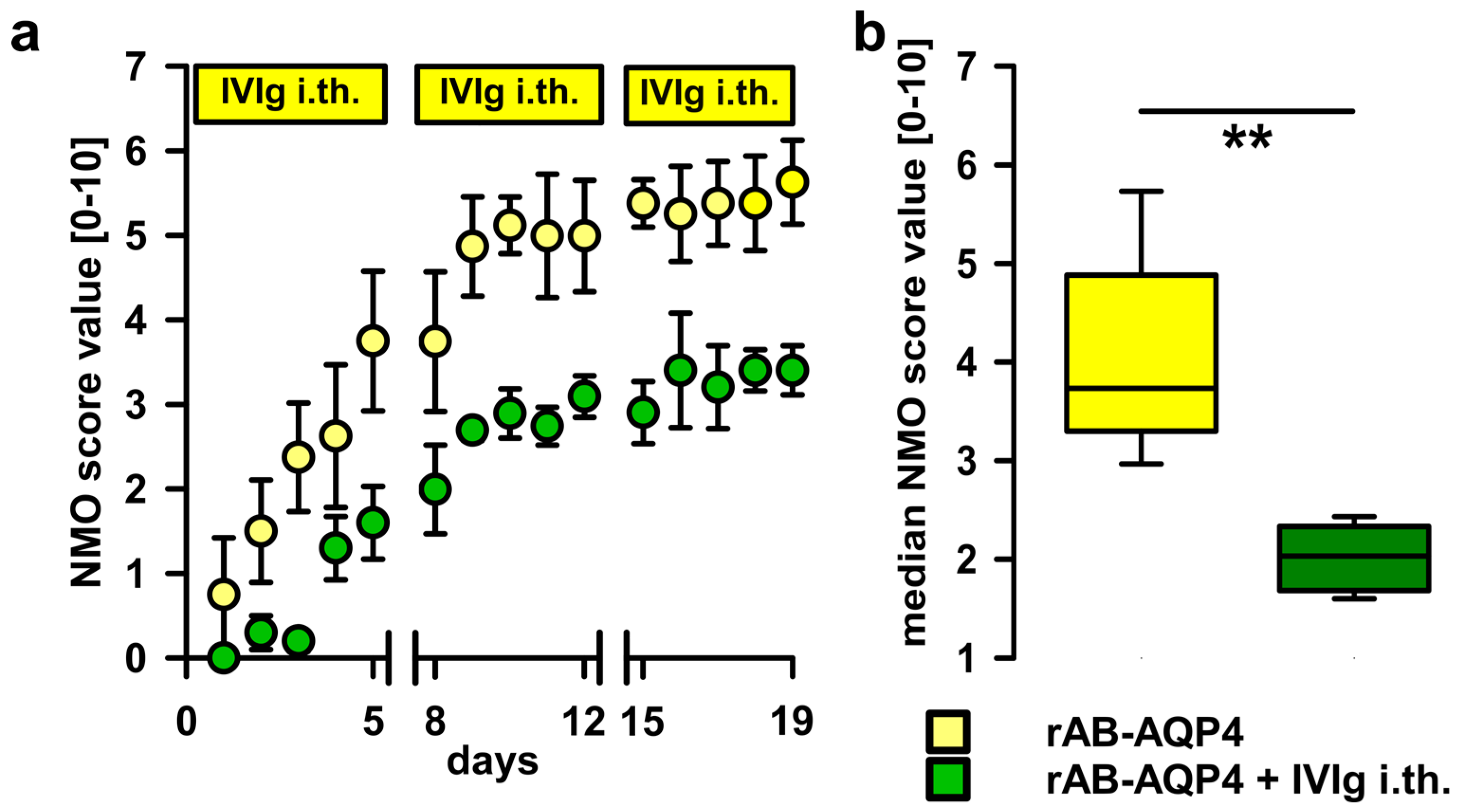
2.3. Intrathecal Co-Administration of IVIg Is Effective in Reducing the Pathogenic Effects of NMO-IgG and of Recombinant Human Autoantibodies to Aquaporin-4 (rAB-AQP4)
3. Discussion
4. Materials and Methods
4.1. Patients, Therapeutic Plasma Exchange, and Preparation of IgG Fractions and rAB-AQP4
4.2. Animals, Surgery and Intrathecal Injections
4.3. Behavioral Analyses
4.4. Data Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| AB | autoantibody |
| AQP4-AB | aquaporin-4 autoantibody |
| CNS | Central Nervous System |
| GBS | Guillain-Barré Syndrome |
| IVIg | pooled human immunoglobulins |
| NMO-IgG | purified immunoglobulin G from patients suffering from NMOSD |
| NMSOD | Neuromyelitis Optica Spectrum Disorder |
| i.p. | intraperitoneal |
| i.th. | intrathecal |
| rAB-AQP4 | recombinant human anti-aquaporin-4 antibody |
References
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; de Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Lennon, V.A.; Wingerchuk, D.M.; Kryzer, T.J.; Pittock, S.J.; Lucchinetti, C.F.; Fujihara, K.; Nakashima, I.; Weinshenker, B.G. A serum autoantibody marker of neuromyelitis optica: Distinction from multiple sclerosis. Lancet 2004, 364, 2106–2112. [Google Scholar] [CrossRef]
- Lennon, V.A.; Kryzer, T.J.; Pittock, S.J.; Verkman, A.S.; Hinson, S.R. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J. Exp. Med. 2005, 202, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Jasiak-Zatonska, M.; Kalinowska-Lyszczarz, A.; Michalak, S.; Kozubski, W. The immunology of neuromyelitis optica-current knowledge, clinical implications, controversies and future perspectives. Int. J. Mol. Sci. 2016, 17, 273. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Lennon, V.A.; Pittock, S.J.; Lucchinetti, C.F.; Weinshenker, B.G. Revised diagnostic criteria for neuromyelitis optica. Neurology 2006, 66, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.T.; Mao, Z.; Qiu, W.; Hu, X.; Wingerchuk, D.M.; Weinshenker, B.G. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2016, 86, 491–492. [Google Scholar] [CrossRef] [PubMed]
- Ratelade, J.; Asavapanumas, N.; Ritchie, A.M.; Wemlinger, S.; Bennett, J.L.; Verkman, A.S. Involvement of antibody-dependent cell-mediated cytotoxicity in inflammatory demyelination in a mouse model of neuromyelitis optica. Acta Neuropathol. 2013, 126, 699–709. [Google Scholar] [CrossRef] [PubMed]
- Bennett, J.L.; Lam, C.; Kalluri, S.R.; Saikali, P.; Bautista, K.; Dupree, C.; Glogowska, M.; Case, D.; Antel, J.P.; Owens, G.P.; et al. Intrathecal pathogenic anti-aquaporin-4 antibodies in early neuromyelitis optica. Ann. Neurol. 2009, 66, 617–629. [Google Scholar] [CrossRef] [PubMed]
- Marignier, R.; Nicolle, A.; Watrin, C.; Touret, M.; Cavagna, S.; Varrin-Doyer, M.; Cavillon, G.; Rogemond, V.; Confavreux, C.; Honnorat, J.; et al. Oligodendrocytes are damaged by neuromyelitis optica immunoglobulin G via astrocyte injury. Brain 2010, 133, 2578–2791. [Google Scholar] [CrossRef] [PubMed]
- Geis, C.; Ritter, C.; Ruschil, C.; Weishaupt, A.; Grunewald, B.; Stoll, G.; Holmoy, T.; Misu, T.; Fujihara, K.; Hemmer, B.; et al. The intrinsic pathogenic role of autoantibodies to aquaporin 4 mediating spinal cord disease in a rat passive-transfer model. Exp. Neurol. 2015, 265, 8–21. [Google Scholar] [CrossRef]
- Misu, T.; Höftberger, R.; Fujihara, K.; Wimmer, I.; Takai, Y.; Nishiyama, S.; Nakashima, I.; Konno, H.; Bradl, M.; Garzuly, F.; et al. Presence of six different lesion types suggests diverse mechanisms of tissue injury in neuromyelitis optica. Acta Neuropathol. 2013, 125, 815–827. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.C.; Bennett, J.L.; Verkman, A.S. Treatment of neuromyelitis optica: State-of-the-art and emerging therapies. Nat. Rev. Neurol. 2014, 10, 493–506. [Google Scholar] [CrossRef] [PubMed]
- Viswanathan, S.; Wong, A.H.; Quek, A.M.; Yuki, N. Intravenous immunoglobulin may reduce relapse frequency in neuromyelitis optica. J. Neuroimmunol. 2015, 282, 92–96. [Google Scholar] [CrossRef] [PubMed]
- Elsone, L.; Panicker, J.; Mutch, K.; Boggild, M.; Appleton, R.; Jacob, A. Role of intravenous immunoglobulin in the treatment of acute relapses of neuromyelitis optica: Experience in 10 patients. Mult. Scler. 2014, 20, 501–504. [Google Scholar] [CrossRef] [PubMed]
- Ratelade, J.; Smith, A.J.; Verkman, A.S. Human immunoglobulin G reduces the pathogenicity of aquaporin-4 autoantibodies in neuromyelitis optica. Exp. Neurol. 2014, 255, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Gold, R.; Stangel, M.; Dalakas, M.C. Drug Insight: The use of intravenous immunoglobulin in neurology—Therapeutic considerations and practical issues. Nat. Clin. Pract. Neurol. 2007, 3, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Hughes, R.A.; Swan, A.V.; van Doorn, P.A. Intravenous immunoglobulin for Guillain-Barre syndrome. Cochrane Database Syst. Rev. 2014. [Google Scholar] [CrossRef]
- Magraner, M.J.; Coret, F.; Casanova, B. The effect of intravenous immunoglobulin on neuromyelitis optica. Neurologia 2013, 28, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Bichuetti, D.; Boulos, F.; Shinosaki, J.; Souza, N.; Oliveira, E.; Gabbai, A. Treatment of Neuromyelitis Optica with Intravenous Immunoglobulin: Report of 8 Patients. Neurology 2012, 78 (Suppl. 1), P04.141. [Google Scholar] [CrossRef]
- Yu, Z.; Lennon, V.A. Mechanism of intravenous immune globulin therapy in antibody-mediated autoimmune diseases. N. Engl. J. Med. 1999, 340, 227–228. [Google Scholar] [CrossRef] [PubMed]
- Dalakas, M.C. Intravenous immunoglobulin in the treatment of autoimmune neuromuscular diseases: Present status and practical therapeutic guidelines. Muscle Nerve 1999, 22, 1479–1497. [Google Scholar] [CrossRef]
- Buchwald, B.; Ahangari, R.; Weishaupt, A.; Toyka, K.V. Intravenous immunoglobulins neutralize blocking antibodies in Guillain-Barre syndrome. Ann. Neurol. 2002, 51, 673–680. [Google Scholar] [CrossRef] [PubMed]
- Buchwald, B.; Ahangari, R.; Weishaupt, A.; Toyka, K.V. Presynaptic effects of immunoglobulin G from patients with Lambert-Eaton myasthenic syndrome: Their neutralization by intravenous immunoglobulins. Muscle Nerve 2005, 31, 487–494. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The arrive guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef] [PubMed]
- Geis, C.; Weishaupt, A.; Hallermann, S.; Grunewald, B.; Wessig, C.; Wultsch, T.; Reif, A.; Byts, N.; Beck, M.; Jablonka, S.; et al. Stiff person syndrome-associated autoantibodies to amphiphysin mediate reduced GABAergic inhibition. Brain 2010, 133, 3166–3180. [Google Scholar] [CrossRef] [PubMed]
- Gabriel, C.M.; Gregson, N.A.; Redford, E.J.; Davies, M.; Smith, K.J.; Hughes, R.A. Human immunoglobulin ameliorates rat experimental autoimmune neuritis. Brain 1997, 120, 1533–1540. [Google Scholar] [CrossRef] [PubMed]
- Enders, U.; Toyka, K.V.; Hartung, H.P.; Gold, R. Failure of intravenous immunoglobulin (IVIg) therapy in experimental autoimmune neuritis (EAN) of the Lewis rat. J. Neuroimmunol. 1997, 76, 112–116. [Google Scholar] [CrossRef]
- Sommer, C.; Weishaupt, A.; Brinkhoff, J.; Biko, L.; Wessig, C.; Gold, R.; Toyka, K.V. Paraneoplastic stiff-person syndrome: Passive transfer to rats by means of IgG antibodies to amphiphysin. Lancet 2005, 365, 1406–1411. [Google Scholar] [CrossRef]
- Linker, R.A.; Maurer, M.; Gaupp, S.; Martini, R.; Holtmann, B.; Giess, R.; Rieckmann, P.; Lassmann, H.; Toyka, K.V.; Sendtner, M.; et al. CNTF is a major protective factor in demyelinating CNS disease: A neurotrophic cytokine as modulator in neuroinflammation. Nat. Med. 2002, 8, 620–624. [Google Scholar] [CrossRef] [PubMed]



| Score | Symptoms |
|---|---|
| 0 | normal |
| 1 | reduced tone of tail |
| 2 | limp tail, impaired righting |
| 3 | absent righting |
| 4 | gait ataxia |
| 5 | mild paraparesis of hindlimb |
| 6 | moderate paraparesis |
| 7 | severe paraparesis or paraplegia |
| 8 | tetraparesis |
| 9 | moribund |
| 10 | death |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grünewald, B.; Bennett, J.L.; Toyka, K.V.; Sommer, C.; Geis, C. Efficacy of Polyvalent Human Immunoglobulins in an Animal Model of Neuromyelitis Optica Evoked by Intrathecal Anti-Aquaporin 4 Antibodies. Int. J. Mol. Sci. 2016, 17, 1407. https://doi.org/10.3390/ijms17091407
Grünewald B, Bennett JL, Toyka KV, Sommer C, Geis C. Efficacy of Polyvalent Human Immunoglobulins in an Animal Model of Neuromyelitis Optica Evoked by Intrathecal Anti-Aquaporin 4 Antibodies. International Journal of Molecular Sciences. 2016; 17(9):1407. https://doi.org/10.3390/ijms17091407
Chicago/Turabian StyleGrünewald, Benedikt, Jeffrey L. Bennett, Klaus V. Toyka, Claudia Sommer, and Christian Geis. 2016. "Efficacy of Polyvalent Human Immunoglobulins in an Animal Model of Neuromyelitis Optica Evoked by Intrathecal Anti-Aquaporin 4 Antibodies" International Journal of Molecular Sciences 17, no. 9: 1407. https://doi.org/10.3390/ijms17091407
APA StyleGrünewald, B., Bennett, J. L., Toyka, K. V., Sommer, C., & Geis, C. (2016). Efficacy of Polyvalent Human Immunoglobulins in an Animal Model of Neuromyelitis Optica Evoked by Intrathecal Anti-Aquaporin 4 Antibodies. International Journal of Molecular Sciences, 17(9), 1407. https://doi.org/10.3390/ijms17091407