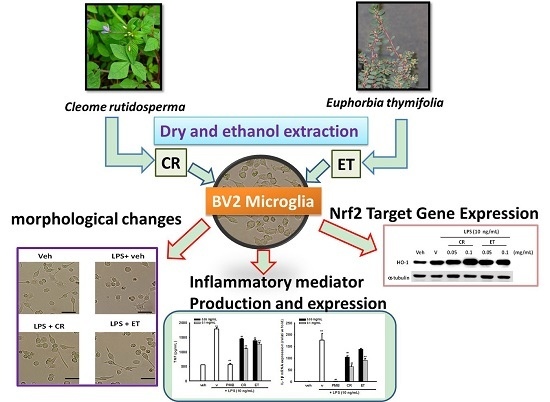
Cleome rutidosperma and Euphorbia thymifolia Suppress Inflammatory Response via Upregulation of Phase II Enzymes and Modulation of NF-κB and JNK Activation in LPS-Stimulated BV2 Microglia
Abstract
:1. Introduction
2. Results
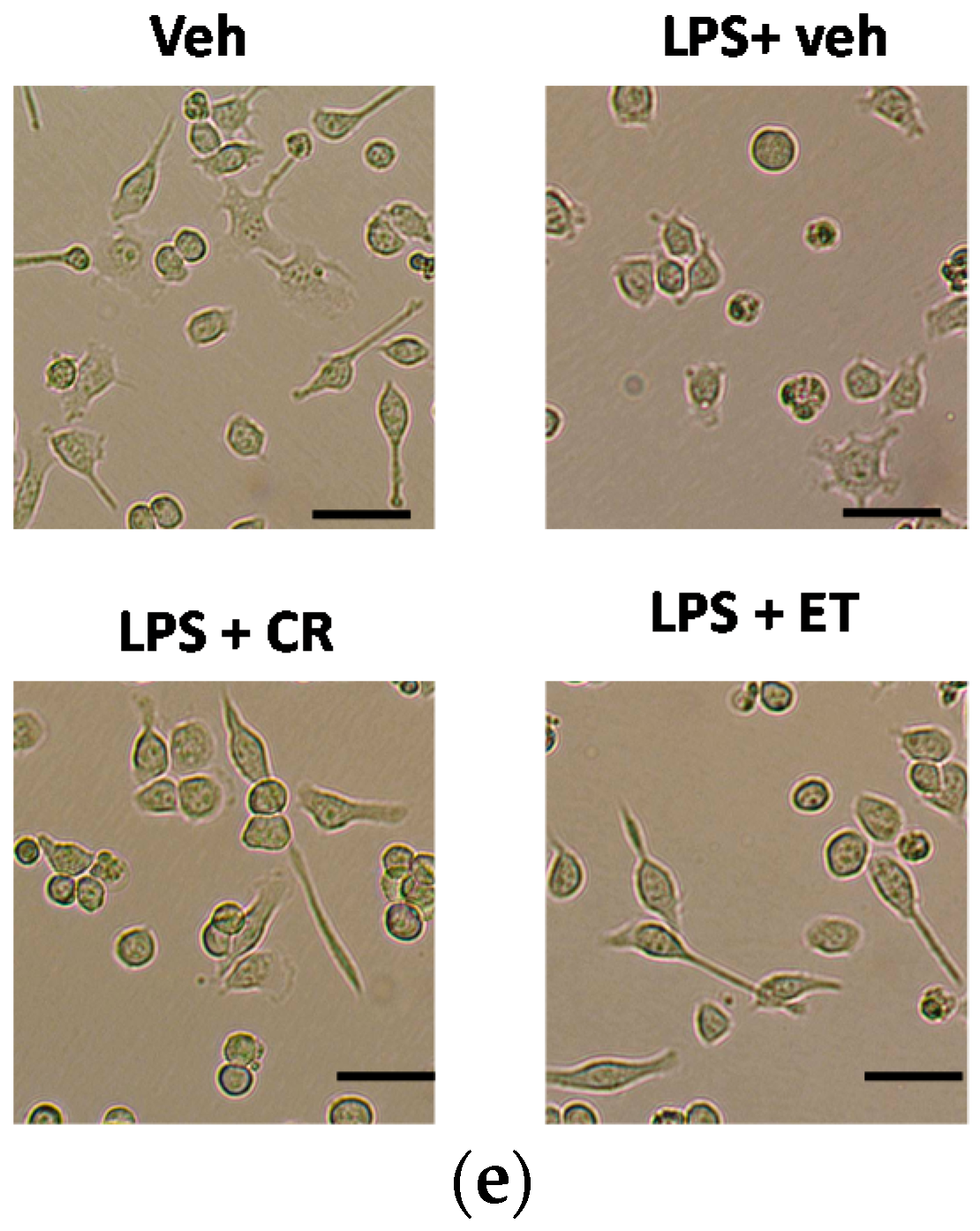
2.1. Ethanol Extracts of C. rutidosperma (CR) and E. thymifolia (ET) Inhibited Nitric Oxide (NO) Production and Activation in Lipopolysaccharide (LPS)-Treated BV2 Cells
2.2. Ethanol Extracts of C. rutidosperma and E. thymifolia Down-Regulated Protein Expression of Inducible Nitric Oxide Synthase (iNOS) and Cyclooxygenase (COX)-2
2.3. Effects of Ethanol Extracts of C. rutidosperma and E. thymifolia on mRNA Expression of iNOS and COX-2
2.4. Effects of Ethanol Extracts of C. rutidosperma and E. thymifolia on Pro-inflammatory Cytokine Production and Expression
2.5. Ethanol Extracts of C. rutidosperma and E. thymifolia Induce Nrf2 Target Gene Expression
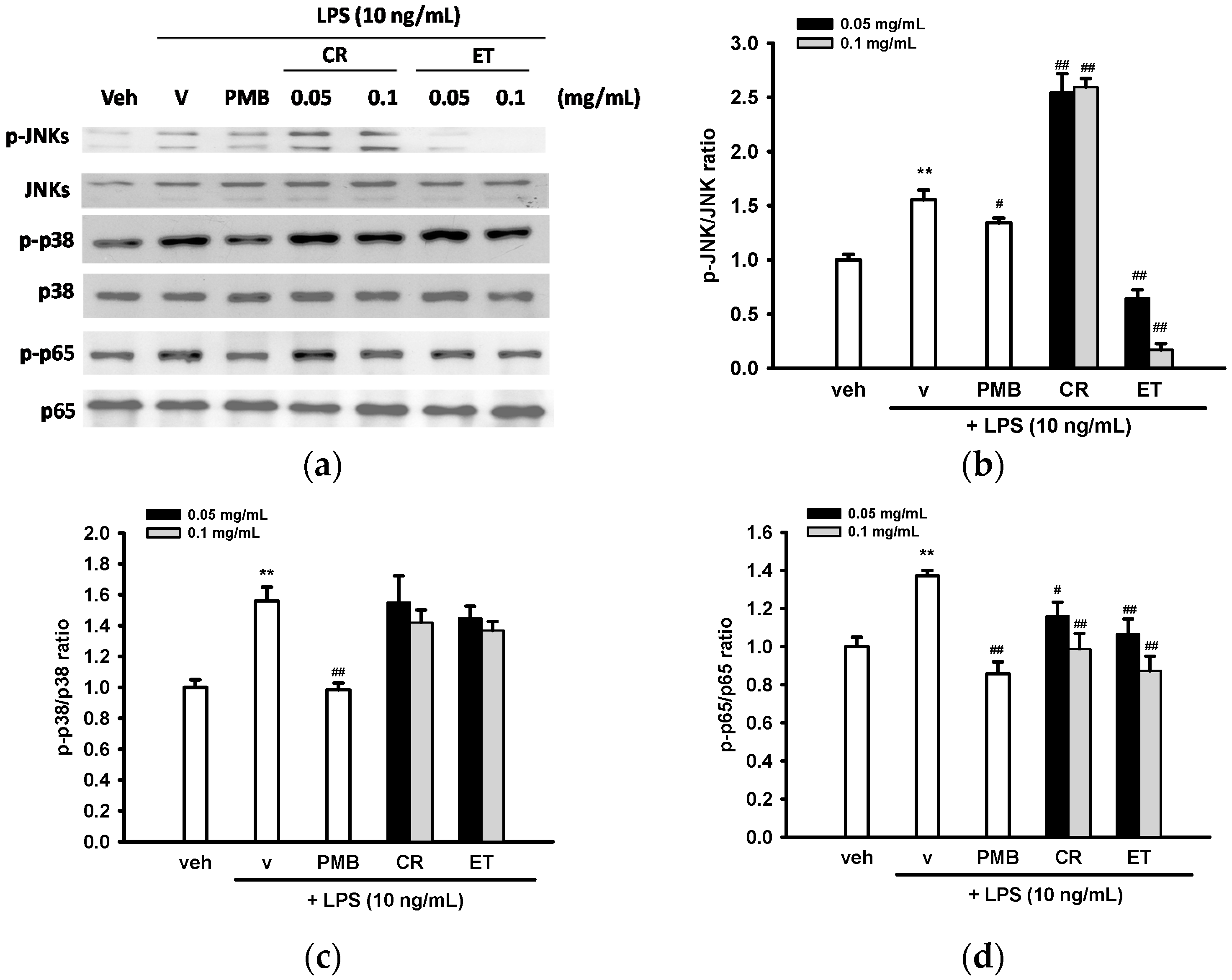
2.6. Effects of Ethanol Extracts of C. rutidosperma and E. thymifolia on LPS-Mediated Activation of MAPK and NF-κB
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Extraction
4.3. BV2 Cell Culture and Measurement of Nitrite, TNF and IL-6 Release
4.4. Cell Viability
4.5. Western Blotting Analysis
4.6. RNA Extraction and Reverse Transcription Real-Time PCR
4.7. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Perry, V.H.; Nicoll, J.A.; Holmes, C. Microglia in neurodegenerative disease. Nat. Rev. Neurol. 2010, 6, 193–201. [Google Scholar] [CrossRef] [PubMed]
- Brown, G.C.; Neher, J.J. Microglial phagocytosis of live neurons. Nat. Rev. Neurosci. 2014, 15, 209–216. [Google Scholar] [CrossRef] [PubMed]
- Liu, B.; Wang, K.; Gao, H.M.; Mandavilli, B.; Wang, J.Y.; Hong, J.S. Molecular consequences of activated microglia in the brain: Overactivation induces apoptosis. J. Neurochem. 2001, 77, 182–189. [Google Scholar] [CrossRef] [PubMed]
- McMahon, M.; Lamont, D.J.; Beattie, K.A.; Hayes, J.D. Keap1 perceives stress via three sensors for the endogenous signaling molecules nitric oxide, zinc, and alkenals. Proc. Natl. Acad. Sci. USA 2010, 107, 18838–18843. [Google Scholar] [CrossRef] [PubMed]
- Magesh, S.; Chen, Y.; Hu, L. Small molecule modulators of keap1-Nrf2-are pathway as potential preventive and therapeutic agents. Med. Res. Rev. 2012, 32, 687–726. [Google Scholar] [CrossRef] [PubMed]
- Maines, M.D. The heme oxygenase system: A regulator of second messenger gases. Annu. Rev. Pharmacol. Toxicol. 1997, 37, 517–554. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Song, W.; Zukor, H.; Hascalovici, J.R.; Zeligman, D. Heme oxygenase-1 and neurodegeneration: Expanding frontiers of engagement. J. Neurochem. 2009, 110, 469–485. [Google Scholar] [CrossRef] [PubMed]
- Scapagnini, G.; Foresti, R.; Calabrese, V.; Giuffrida Stella, A.M.; Green, C.J.; Motterlini, R. Caffeic acid phenethyl ester and curcumin: A novel class of heme oxygenase-1 inducers. Mol. Pharmacol. 2002, 61, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Lee, S.S.; Chen, S.C.; Ho, F.M.; Lin, W.W. Oregonin inhibits lipopolysaccharide-induced inos gene transcription and upregulates HO-1 expression in macrophages and microglia. Br. J. Pharmacol. 2005, 146, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Park, S.Y.; Kim, Y.H.; Kim, E.K.; Ryu, E.Y.; Lee, S.J. Heme oxygenase-1 signals are involved in preferential inhibition of pro-inflammatory cytokine release by surfactin in cells activated with porphyromonas gingivalis lipopolysaccharide. Chem. Biol. Interact. 2010, 188, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Chiou, S.Y.; Shen, Y.T.; Yen, F.T.; Ding, H.Y.; Wu, M.J. Anti-inflammatory effect and mechanism of the green fruit extract of solanum integrifolium poir. BioMed Res. Int. 2014, 2014, 953873. [Google Scholar] [PubMed]
- Chiou, S.Y.; Ha, C.L.; Wu, P.S.; Yeh, C.L.; Su, Y.S.; Li, M.P.; Wu, M.J. Antioxidant, anti-tyrosinase and anti-inflammatory activities of oil production residues from camellia tenuifloria. Int. J. Mol. Sci. 2015, 16, 29522–29541. [Google Scholar] [CrossRef] [PubMed]
- Pearson, G.; Robinson, F.; Beers Gibson, T.; Xu, B.E.; Karandikar, M.; Berman, K.; Cobb, M.H. Mitogen-activated protein (MAP) kinase pathways: Regulation and physiological functions. Endocr. Rev. 2001, 22, 153–183. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.L.; Lapadat, R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science 2002, 298, 1911–1912. [Google Scholar] [CrossRef] [PubMed]
- Weston, C.R.; Davis, R.J. The JNK signal transduction pathway. Curr. Opin. Cell Biol. 2007, 19, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Natoli, G.; De Santa, F. Shaping alternative NF-κB-dependent gene expression programs: New clues to specificity. Cell Death Differ. 2006, 13, 693–696. [Google Scholar] [CrossRef] [PubMed]
- Viatour, P.; Merville, M.P.; Bours, V.; Chariot, A. Phosphorylation of NF-κB and IκB proteins: Implications in cancer and inflammation. Trends Biochem. Sci. 2005, 30, 43–52. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, H.; Chiba, H.; Miyoshi, H.; Sugita, T.; Toriumi, W. IκB kinases phosphorylate NF-κB p65 subunit on serine 536 in the transactivation domain. J. Biol. Chem. 1999, 274, 30353–30356. [Google Scholar] [CrossRef] [PubMed]
- Choi, D.K.; Koppula, S.; Suk, K. Inhibitors of microglial neurotoxicity: Focus on natural products. Molecules 2011, 16, 1021–1043. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, A.; Roy, H. Evaluation of anti-arthritic activity of ethanolic extract of Cleome rutidosperma. J. Pharm. Sci. Technol. 2010, 2, 330–332. [Google Scholar]
- Bose, A.; Gupta, J.; Dash, G.; Ghosh, T.; Si, S.; Panda, D. Diuretic and antibacterial activity of aqueous extract of Cleome rutidosperma DC. Indian J. Pharm. Sci. 2007, 69, 292–294. [Google Scholar] [CrossRef]
- Bose, A.; Mondal, S.; Gupta, J.; Ghosh, T.; Si, S.; Debbhuti, D. A study on antimicrobial activity of Cleome rutidosperma DC. J. Nat. Rem. 2007, 7, 132–134. [Google Scholar]
- Bose, A.; Mondal, S.; Gupta, J.K.; Ghosh, T.; Dash, G.K.; Si, S. Analgesic, anti-inflammatory and antipyretic activities of the ethanolic extract and its fractions of Cleome rutidosperma. Fitoterapia 2007, 78, 515–520. [Google Scholar] [CrossRef] [PubMed]
- Bose, A.; Mondal, S.; Gupta, J.K.; Ghosh, T.; Debbhuti, D.; Si, S. Antioxidant and free radical scavenging activities of Cleome rutidosperma. Orient Pharm. Exp. Med. 2008, 8, 135–145. [Google Scholar] [CrossRef]
- Mondal, S.; Suresh, P. Wound healing activity of Cleome rutidosperma DC. Roots. Int. Curr. Pharm. J. 2012, 1, 151–154. [Google Scholar] [CrossRef]
- Mali, P.Y.; Panchal, S.S. A review on phyto-pharmacological potentials of Euphorbia thymifolia L. Anc. Sci. Life 2013, 32, 165–172. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.C.; Cheng, H.Y.; Yang, C.M.; Lin, T.C. Antioxidant and antiviral activities of Euphorbia thymifolia L. J. Biomed. Sci. 2002, 9, 656–664. [Google Scholar] [CrossRef] [PubMed]
- Garipelli, N.; Runja, C.; Potnuri, N.; Pigili, R.K. Anti-inflammatory and anti-oxidant activities of ethanolic extract of Euphorbia thymifolia linn whole plant. Int. J. Pharm. Pharm. Sci. 2012, 4, 516–519. [Google Scholar]
- Amaral, A.; Kuster, R.; Gonçalves, J.; Wigg, M. Antiviral investigation on the flavonoids of Chamaesyce thymifolia. Fitoterapia 1999, 70, 293–295. [Google Scholar] [CrossRef]
- Yang, C.M.; Cheng, H.Y.; Lin, T.C.; Chiang, L.C.; Lin, C.C. Euphorbia thymifolia suppresses herpes simplex virus-2 infection by directly inactivating virus infectivity. Clin. Exp. Pharmacol. Physiol. 2005, 32, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Khan, N.; Rahman, M.; Nur-e-Kamal, M. Antibacterial activity of Euphorbia thymifolia linn. Indian J. Med. Res. 1988, 87, 395–397. [Google Scholar] [PubMed]
- Kane, S.R.; Mohite, S.K.; Shete, J.S. Antihelmintic activity of aqueous and methanolic extracts of Euphorbia thymifolia linn. Int. J. Pharm. Tech. Res. 2009, 1, 666–669. [Google Scholar]
- Henn, A.; Lund, S.; Hedtjarn, M.; Schrattenholz, A.; Porzgen, P.; Leist, M. The suitability of BV2 cells as alternative model system for primary microglia cultures or for animal experiments examining brain inflammation. Altex 2009, 26, 83–94. [Google Scholar] [PubMed]
- Fricker, M.; Oliva-Martin, M.J.; Brown, G.C. Primary phagocytosis of viable neurons by microglia activated with LPS or abeta is dependent on calreticulin/lrp phagocytic signalling. J. Neuroinflamm. 2012, 9, 196. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.J.; Wang, L.; Ding, H.Y.; Weng, C.Y.; Yen, J.H. Glossogyne tenuifolia acts to inhibit inflammatory mediator production in a macrophage cell line by downregulating LPS-induced NF-κB. J. Biomed. Sci. 2004, 11, 186–199. [Google Scholar] [CrossRef] [PubMed]
- Stansley, B.; Post, J.; Hensley, K. A comparative review of cell culture systems for the study of microglial biology in Alzheimer’s disease. J. Neuroinflamm. 2012, 9, 115. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.S.; Mann, M.; DuBois, R.N. The role of cyclooxygenases in inflammation, cancer, and development. Oncogene 1999, 18, 7908–7916. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.Y.; Tan, M.S.; Yu, J.T.; Tan, L. Role of pro-inflammatory cytokines released from microglia in Alzheimer’s disease. Ann. Trans. Med. 2015, 3, 136. [Google Scholar] [CrossRef]
- Bose, S.; Kim, S.; Oh, Y.; Moniruzzaman, M.; Lee, G.; Cho, J. Effect of CCL2 on BV2 microglial cell migration: Involvement of probable signaling pathways. Cytokine 2016, 81, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.L.; Ho, Y.C.; Lin, C.Y.; Yet, S.F. Heme oxygenase-1 in inflammation and cardiovascular disease. Am. J. Cardiovasc. Dis. 2011, 1, 150–158. [Google Scholar] [PubMed]
- Lin, H.Y.; Juan, S.H.; Shen, S.C.; Hsu, F.L.; Chen, Y.C. Inhibition of lipopolysaccharide-induced nitric oxide production by flavonoids in RAW264.7 macrophages involves heme oxygenase-1. Biochem. Pharmacol. 2003, 66, 1821–1832. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, H.K.; Shin, J.H.; Lee, J.K. Up-down regulation of HO-1 and inos gene expressions by ethyl pyruvate via recruiting p300 to Nrf2 and depriving it from p65. Free Radic. Biol. Med. 2013, 65, 468–476. [Google Scholar] [CrossRef] [PubMed]
- Saklatvala, J.; Dean, J.; Clark, A. Control of the expression of inflammatory response genes. Biochem. Soc. Symp. 2003, 95–106. [Google Scholar] [CrossRef]
- Kingwell, K. Neurodegenerative disease: Microglia in early disease stages. Nat. Rev. Neurol. 2012, 8, 475. [Google Scholar] [CrossRef] [PubMed]
- Jang, S.; Kelley, K.W.; Johnson, R.W. Luteolin reduces IL-6 production in microglia by inhibiting JNK phosphorylation and activation of AP-1. Proc. Natl. Acad. Sci. USA 2008, 105, 7534–7539. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Yamamoto, M. Nrf2-keap1 regulation of cellular defense mechanisms against electrophiles and reactive oxygen species. Adv. Enzym. Regul. 2006, 46, 113–140. [Google Scholar] [CrossRef] [PubMed]
- Alcaraz, M.J.; Fernandez, P.; Guillen, M.I. Anti-inflammatory actions of the heme oxygenase-1 pathway. Curr. Pharm. Des. 2003, 9, 2541–2551. [Google Scholar] [CrossRef] [PubMed]
- Clark, A.R.; Dean, J.L.; Saklatvala, J. The p38 mapk pathway mediates both antiinflammatory and proinflammatory processes: Comment on the article by damjanov and the editorial by genovese. Arthritis Rheum. 2009, 60, 3513–3514. [Google Scholar] [CrossRef] [PubMed]
- Dai, Y.; Chen, S.; Wang, L.; Pei, X.Y.; Funk, V.L.; Kramer, L.B.; Dent, P.; Grant, S. Disruption of IκB kinase (IKK)-mediated rela serine 536 phosphorylation sensitizes human multiple myeloma cells to histone deacetylase (HDAC) inhibitors. J. Biol. Chem. 2011, 286, 34036–34050. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, M.L.; Mattioli, I.; Buss, H.; Kracht, M. NF-κB: A multifaceted transcription factor regulated at several levels. Chembiochem 2004, 5, 1348–1358. [Google Scholar] [CrossRef] [PubMed]
- Buelna-Chontal, M.; Zazueta, C. Redox activation of Nrf2 & NF-κB : A double end sword? Cell Signal. 2013, 25, 2548–2557. [Google Scholar] [PubMed]
- Li, W.; Khor, T.O.; Xu, C.; Shen, G.; Jeong, W.S.; Yu, S.; Kong, A.N. Activation of Nrf2-antioxidant signaling attenuates NF-κB-inflammatory response and elicits apoptosis. Biochem. Pharmacol. 2008, 76, 1485–1489. [Google Scholar] [CrossRef] [PubMed]
- Blasi, E.; Barluzzi, R.; Bocchini, V.; Mazzolla, R.; Bistoni, F. Immortalization of murine microglial cells by a v-raf/v-myc carrying retrovirus. J. Neuroimmunol. 1990, 27, 229–237. [Google Scholar] [CrossRef]
- Carmichael, J.; DeGraff, W.G.; Gazdar, A.F.; Minna, J.D.; Mitchell, J.B. Evaluation of a tetrazolium-based semiautomated colorimetric assay: Assessment of chemosensitivity testing. Cancer Res. 1987, 47, 936–942. [Google Scholar] [PubMed]






| Antibody | Company | Catalog Number |
|---|---|---|
| α-tubulin | Sigma-Aldrich | T6199 |
| NOS | Cell Signaling (Danvers, MA, USA) | 2977 |
| COX-2 | Santa Cruz (Santa Cruz, CA, USA) | Sc-166475 |
| HO-1 | Stressgen (San Diego, CA, USA) | SPA-895 |
| Phospho-JNK1/2 (clone 81E11) | Cell Signaling | 4668 |
| JNK2 (clone 56G8) | Cell Signaling | 9258 |
| Phoshpo-p38 MAPK(clone 3D7) | Cell Signaling | 9215 |
| p38 MAPK | Cell Signaling | 9212 |
| Phospho-p65 NF-κB (clone 93H1) | Cell Signaling | 3033 |
| p65 NF-κB | Cell Signaling | 3034 |
| Gene | Primers (5′–3′) | Amplicon (bp) |
|---|---|---|
| β-actin | GGCTGTATTCCCCTCCATCG CCAGTTGGTAACAATGCCATGT | 154 |
| iNOS | GTTCTCAGCCCAACAATACAAGA GTGGACGGGTCGATGTCAC | 127 |
| COX-2 | TGAGCAACTATTCCAAACCAGC GCACGTAGTCTTCGATCACTATC | 74 |
| IL-1β | TTCAGGCAGGCAGTATCACTC GAAGGTCCACGGGAAAGACAC | 75 |
| CCL2 | TTAAAAACCTGGATCGGAACCAA GCATTAGCTTCAGATTTACGGGT | 121 |
| HO-1 | AAGCCGAGAATGCTGAGTTCA GCCGTGTAGATATGGTACAAGGA | 100 |
| GCLM | AAGCCCAGGATTGGGTGCCG GGTCGGTGAGCTGTGGGTGT | 141 |
| NQO1 | AGGATGGGAGGTACTCGAATC TGCTAGAGATGACTCGGAAGG | 127 |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, H.-Y.; Wu, P.-S.; Wu, M.-J. Cleome rutidosperma and Euphorbia thymifolia Suppress Inflammatory Response via Upregulation of Phase II Enzymes and Modulation of NF-κB and JNK Activation in LPS-Stimulated BV2 Microglia. Int. J. Mol. Sci. 2016, 17, 1420. https://doi.org/10.3390/ijms17091420
Ding H-Y, Wu P-S, Wu M-J. Cleome rutidosperma and Euphorbia thymifolia Suppress Inflammatory Response via Upregulation of Phase II Enzymes and Modulation of NF-κB and JNK Activation in LPS-Stimulated BV2 Microglia. International Journal of Molecular Sciences. 2016; 17(9):1420. https://doi.org/10.3390/ijms17091420
Chicago/Turabian StyleDing, Hsiou-Yu, Pei-Shan Wu, and Ming-Jiuan Wu. 2016. "Cleome rutidosperma and Euphorbia thymifolia Suppress Inflammatory Response via Upregulation of Phase II Enzymes and Modulation of NF-κB and JNK Activation in LPS-Stimulated BV2 Microglia" International Journal of Molecular Sciences 17, no. 9: 1420. https://doi.org/10.3390/ijms17091420