MicroRNA-378 Alleviates Cerebral Ischemic Injury by Negatively Regulating Apoptosis Executioner Caspase-3
Abstract
:1. Introduction
2. Results
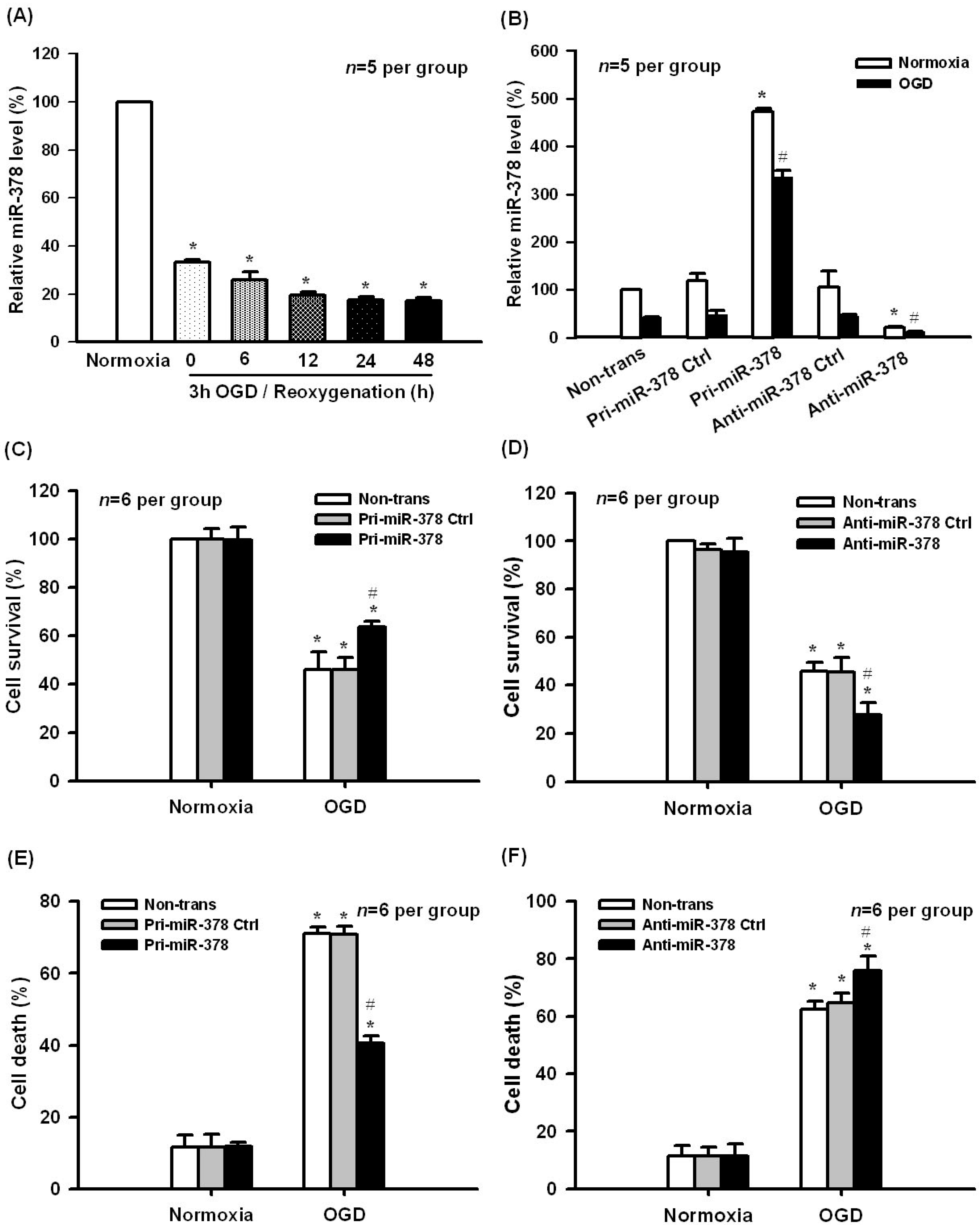
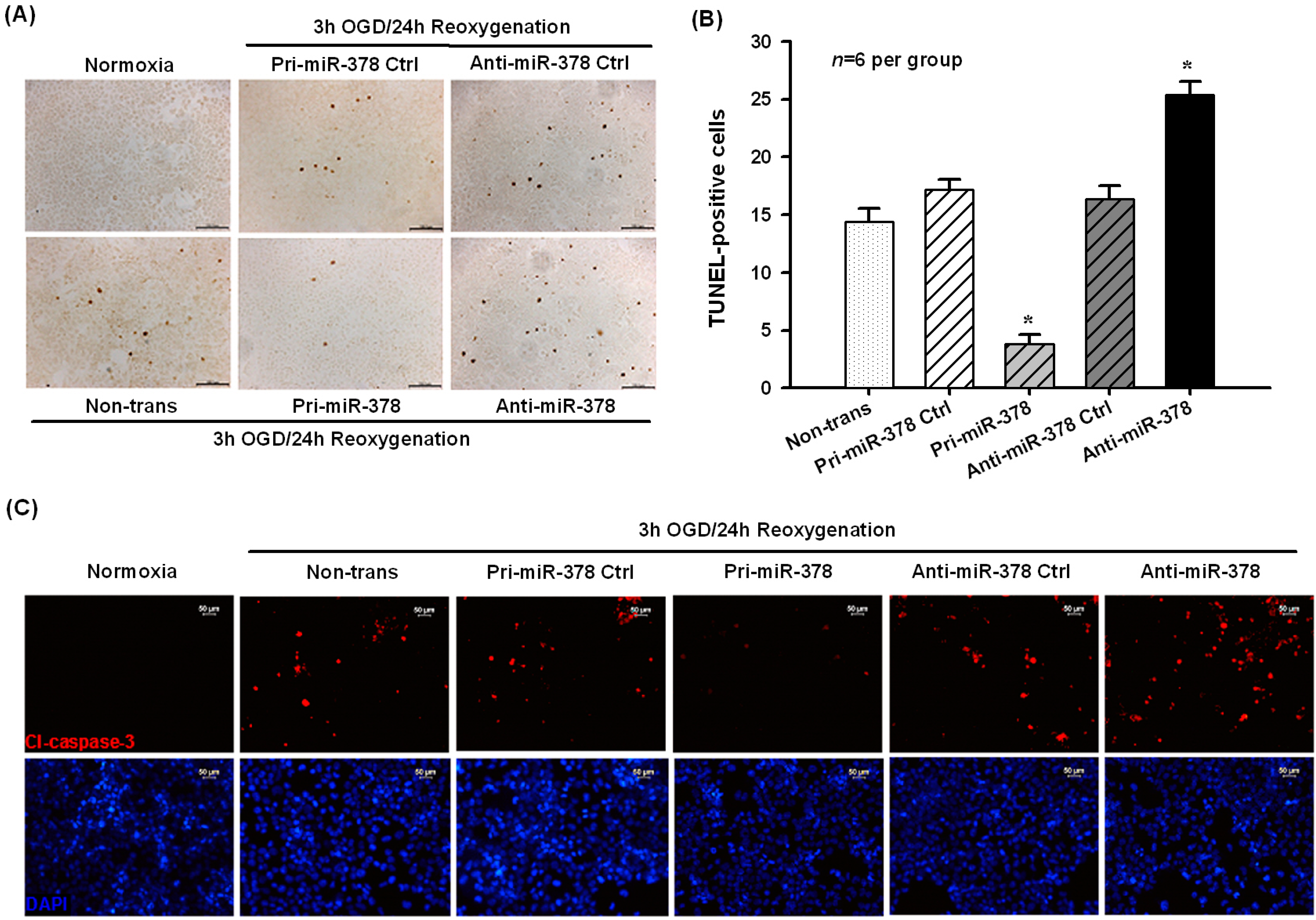
2.1. Effect of miR-378 on OGD-Induced Ischemic Injury in N2A Cells
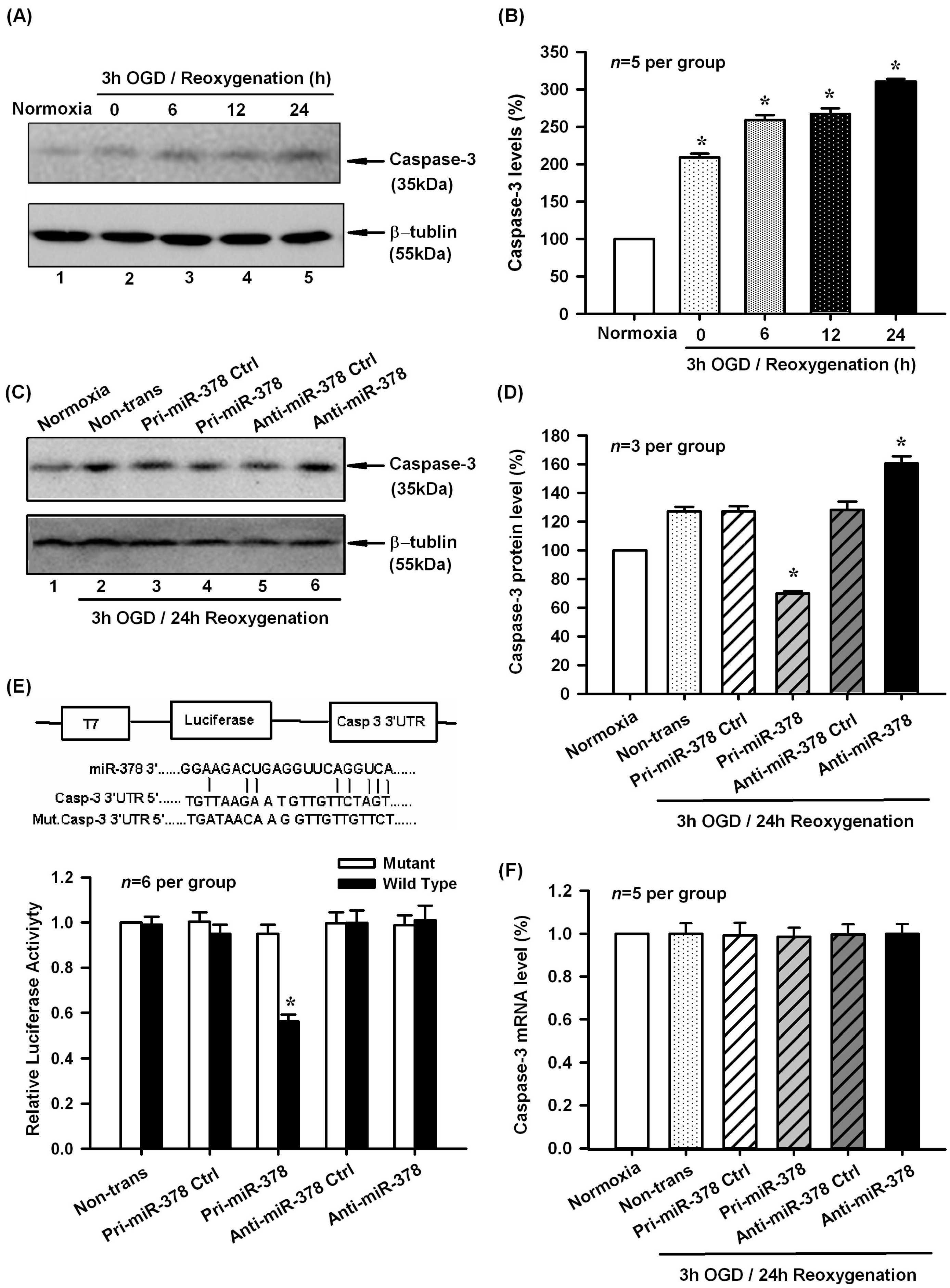
2.2. Effect of miR-378 on the mRNA and Protein Expression Levels of Its Target Gene Caspase-3
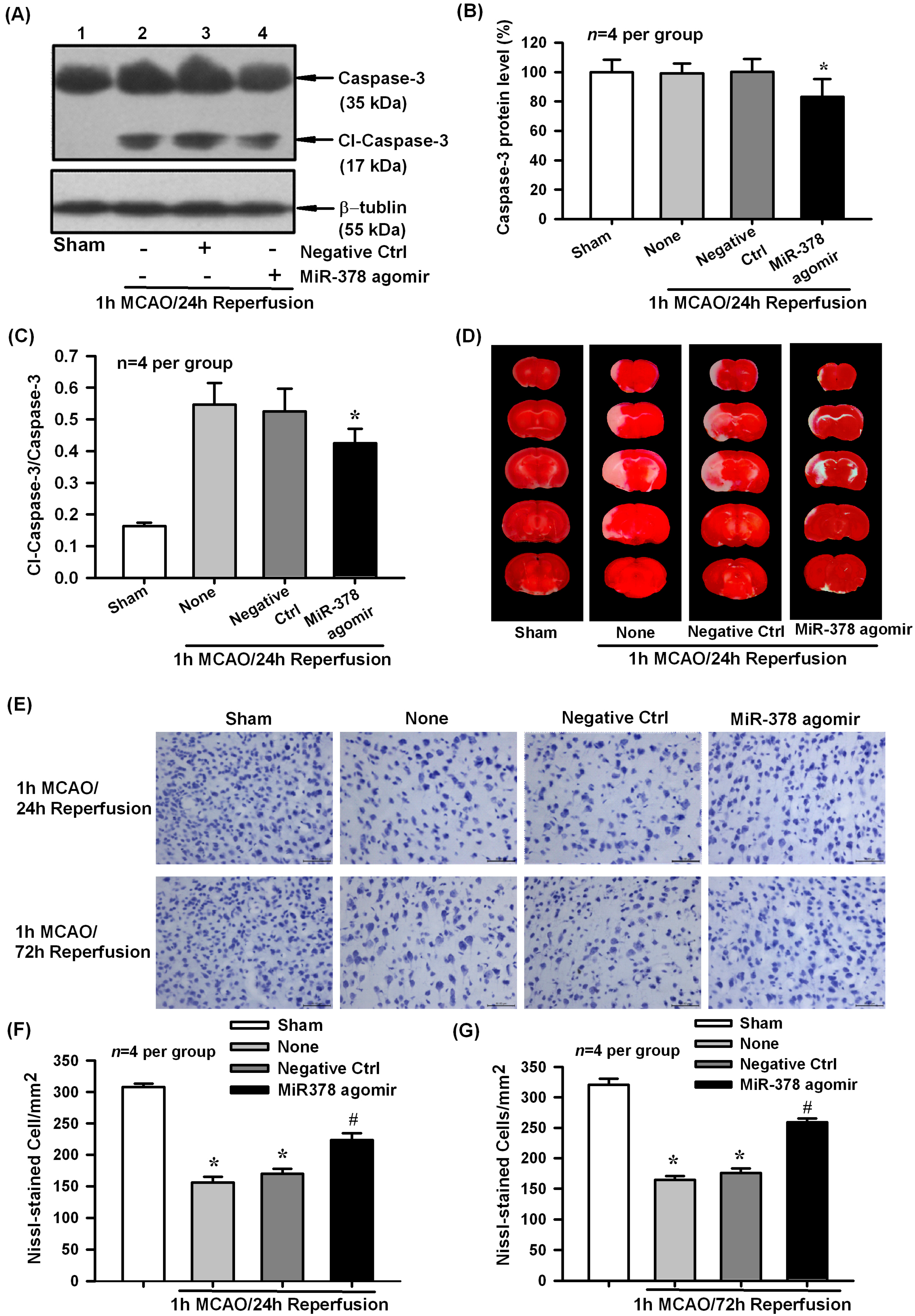
2.3. Effect of miR-378 Overexpression on Transient Focal Cerebral Ischemic Injury of Mice
2.4. Caspase-3 Knockdown Blocked Anti-miR-378-Mediated Neuronal Injury in N2A Cells
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Middle Cerebral Artery Occluded (MCAO)-Induced Transient Focal Cerebral Ischemic Stroke Model
4.3. OGD-Induced Ischemic Injury of N2A Cells
4.4. Plasmid Construction and Luciferase Reporter Assays
4.5. Western Blot Analysis
4.6. Isolation of Total RNA and Quantitative Real-Time RT-PCR Analyses
4.7. TUNEL, Immunofluorescent, TTC, and Nissl Staining
4.8. Statistical Analysis
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Mozaffarian, D.; Benjamin, E.J.; Go, A.S.; Arnett, D.K.; Blaha, M.J.; Cushman, M.; Das, S.R.; de Ferranti, S.; Despres, J.P.; Fullerton, H.J.; et al. Heart disease and stroke statistics-2016 update: A report from the american heart association. Circulation 2016, 133, e38–e60. [Google Scholar] [CrossRef] [PubMed]
- Marsh, J.D.; Keyrouz, S.G. Stroke prevention and treatment. J. Am. Coll. Cardiol. 2010, 56, 683–691. [Google Scholar] [CrossRef] [PubMed]
- Lees, K.R.; Bluhmki, E.; von Kummer, R.; Brott, T.G.; Toni, D.; Grotta, J.C.; Albers, G.W.; Kaste, M.; Marler, J.R.; Hamilton, S.A.; et al. Time to treatment with intravenous alteplase and outcome in stroke: An updated pooled analysis of ecass, atlantis, ninds, and epithet trials. Lancet 2010, 375, 1695–1703. [Google Scholar] [CrossRef]
- Rami, A.; Kogel, D. Apoptosis meets autophagy-like cell death in the ischemic penumbra: Two sides of the same coin? Autophagy 2008, 4, 422–426. [Google Scholar] [CrossRef] [PubMed]
- Fayaz, S.M.; Suvanish Kumar, V.S.; Rajanikant, G.K. Necroptosis: Who knew there were so many interesting ways to die? CNS Neurol. Disord. Drug Targets 2014, 13, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bartel, D.P. MicroRNAs: Genomics, biogenesis, mechanism, and function. Cell 2004, 116, 281–297. [Google Scholar] [CrossRef]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Hammond, S.M. An overview of microRNAs. Adv. Drug Deliv. Rev. 2015, 87, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Delay, C.; Mandemakers, W.; Hebert, S.S. MicroRNAs in Alzheimer’s disease. Neurobiol. Dis. 2012, 46, 285–290. [Google Scholar] [CrossRef] [PubMed]
- Femminella, G.D.; Ferrara, N.; Rengo, G. The emerging role of microRNAs in Alzheimer’s disease. Front. Physiol. 2015, 6, 40. [Google Scholar] [CrossRef] [PubMed]
- Mouradian, M.M. MicroRNAs in Parkinson’s disease. Neurobiol. Dis. 2012, 46, 279–284. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhou, J.; Zhong, Y.; Jiang, L.; Mu, P.; Li, Y.; Singh, N.; Nagarkatti, M.; Nagarkatti, P. Expression, regulation and function of microRNAs in multiple sclerosis. Int. J. Med. Sci. 2014, 11, 810–818. [Google Scholar] [CrossRef] [PubMed]
- Bhalala, O.G.; Srikanth, M.; Kessler, J.A. The emerging roles of microRNAs in CNS injuries. Nat. Rev. Neurol. 2013, 9, 328–339. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.Z.; Tian, Y.; Ander, B.P.; Xu, H.; Stamova, B.S.; Zhan, X.; Turner, R.J.; Jickling, G.; Sharp, F.R. Brain and blood microRNA expression profiling of ischemic stroke, intracerebral hemorrhage, and kainate seizures. J. Cereb. Blood Flow Metab. 2010, 30, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Peng, Z.; Zhang, N.; Yu, L.; Han, S.; Li, D.; Li, J. Identification of differentially expressed microRNAs and their PKC-isoform specific gene network prediction during hypoxic pre-conditioning and focal cerebral ischemia of mice. J. Neurochem. 2012, 120, 830–841. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Song, X.W.; Tian, J.; Chen, H.Y.; Li, D.F.; Wang, J.F.; Ren, A.J.; Yuan, W.J.; Lin, L. Overexpression of microRNA-378 attenuates ischemia-induced apoptosis by inhibiting caspase-3 expression in cardiac myocytes. Apoptosis Int. J. Program. Cell Death 2012, 17, 410–423. [Google Scholar] [CrossRef] [PubMed]
- Dirnagl, U.; Iadecola, C.; Moskowitz, M.A. Pathobiology of ischaemic stroke: An integrated view. Trends Neurosci. 1999, 22, 391–397. [Google Scholar] [CrossRef]
- Doyle, K.P.; Simon, R.P.; Stenzel-Poore, M.P. Mechanisms of ischemic brain damage. Neuropharmacology 2008, 55, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Friedlander, R.M. Apoptosis and caspases in neurodegenerative diseases. N. Engl. J. Med. 2003, 348, 1365–1375. [Google Scholar] [CrossRef] [PubMed]
- Porter, A.G.; Janicke, R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999, 6, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Ni, J.; Wang, X.; Chen, S.; Liu, H.; Wang, Y.; Xu, X.; Cheng, J.; Jia, J.; Zhen, X. MicroRNA let-7c-5p protects against cerebral ischemia injury via mechanisms involving the inhibition of microglia activation. Brain Behav. Immun. 2015, 49, 75–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Wu, M.; Zhao, P.; Huang, Y.; Wang, W.; Yin, W. Neuroprotective effects of viral overexpression of microRNA-22 in rat and cell models of cerebral ischemia-reperfusion injury. J. Cell. Biochem. 2015, 116, 233–241. [Google Scholar] [CrossRef] [PubMed]
- Ham, O.; Lee, S.Y.; Lee, C.Y.; Park, J.H.; Lee, J.; Seo, H.H.; Cha, M.J.; Choi, E.; Kim, S.; Hwang, K.C. Let-7b suppresses apoptosis and autophagy of human mesenchymal stem cells transplanted into ischemia/reperfusion injured heart 7by targeting caspase-3. Stem Cell Res. Ther. 2015, 6, 147. [Google Scholar] [CrossRef] [PubMed]
- Lim, K.Y.; Chua, J.H.; Tan, J.R.; Swaminathan, P.; Sepramaniam, S.; Armugam, A.; Wong, P.T.; Jeyaseelan, K. MicroRNAs in cerebral ischemia. Transl. Stroke Res. 2010, 1, 287–303. [Google Scholar] [CrossRef] [PubMed]
- Sorensen, S.S.; Nygaard, A.B.; Nielsen, M.Y.; Jensen, K.; Christensen, T. MiRNA expression profiles in cerebrospinal fluid and blood of patients with acute ischemic stroke. Transl. Stroke Res. 2014, 5, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Nagalingam, R.S.; Sundaresan, N.R.; Noor, M.; Gupta, M.P.; Solaro, R.J.; Gupta, M. Deficiency of cardiomyocyte-specific microRNA-378 contributes to the development of cardiac fibrosis involving a transforming growth factor β (TGFΒ1)-dependent paracrine mechanism. J. Biol. Chem. 2014, 289, 27199–27214. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Liu, X.; Wang, Y. MicroRNA-378 regulates neural stem cell proliferation and differentiation in vitro by modulating tailless expression. Biochem. Biophys. Res. Commun. 2015, 466, 214–220. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.Y.; Deng, Z.; Wang, C.H.; Yang, B.B. MicroRNA-378 promotes cell survival, tumor growth, and angiogenesis by targeting sufu and FUS-1 expression. Proc. Natl. Acad. Sci. USA 2007, 104, 20350–20355. [Google Scholar] [CrossRef] [PubMed]
- Ma, T.; Jiang, H.; Gao, Y.; Zhao, Y.; Dai, L.; Xiong, Q.; Xu, Y.; Zhao, Z.; Zhang, J. Microarray analysis of differentially expressed microRNAs in non-regressed and regressed bovine corpus luteum tissue; microRNA-378 may suppress luteal cell apoptosis by targeting the interferon γ receptor 1 gene. J. Appl. Genet. 2011, 52, 481–486. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, N.; Liang, J.; Li, J.; Han, S.; Li, J. Micro-RNA-30a regulates ischemia-induced cell death by targeting heat shock protein HSPA5 in primary cultured cortical neurons and mouse brain after stroke. J. Neurosci. Res. 2015, 93, 1756–1768. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Zhang, X.; Li, Y.; Han, S.; Howells, D.W.; Li, S.; Li, J. Conventional protein kinase Cβ-mediated phosphorylation inhibits collapsin response-mediated protein 2 proteolysis and alleviates ischemic injury in cultured cortical neurons and ischemic stroke-induced mice. J. Neurochem. 2016, 137, 446–459. [Google Scholar] [CrossRef] [PubMed]
- Peng, Z.; Li, J.; Li, Y.; Yang, X.; Feng, S.; Han, S.; Li, J. Downregulation of mir-181b in mouse brain following ischemic stroke induces neuroprotection against ischemic injury through targeting heat shock protein A5 and ubiquitin carboxyl-terminal hydrolase isozyme L1. J. Neurosci. Res. 2013, 91, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Krutzfeldt, J.; Rajewsky, N.; Braich, R.; Rajeev, K.G.; Tuschl, T.; Manoharan, M.; Stoffel, M. Silencing of microRNAs in vivo with “antagomirs”. Nature 2005, 438, 685–689. [Google Scholar] [CrossRef] [PubMed]
- Dharap, A.; Bowen, K.; Place, R.; Li, L.C.; Vemuganti, R. Transient focal ischemia induces extensive temporal changes in rat cerebral microRNAome. J. Cereb. Blood Flow Metab. 2009, 29, 675–687. [Google Scholar] [CrossRef] [PubMed]

© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, N.; Zhong, J.; Han, S.; Li, Y.; Yin, Y.; Li, J. MicroRNA-378 Alleviates Cerebral Ischemic Injury by Negatively Regulating Apoptosis Executioner Caspase-3. Int. J. Mol. Sci. 2016, 17, 1427. https://doi.org/10.3390/ijms17091427
Zhang N, Zhong J, Han S, Li Y, Yin Y, Li J. MicroRNA-378 Alleviates Cerebral Ischemic Injury by Negatively Regulating Apoptosis Executioner Caspase-3. International Journal of Molecular Sciences. 2016; 17(9):1427. https://doi.org/10.3390/ijms17091427
Chicago/Turabian StyleZhang, Nan, Jie Zhong, Song Han, Yun Li, Yanling Yin, and Junfa Li. 2016. "MicroRNA-378 Alleviates Cerebral Ischemic Injury by Negatively Regulating Apoptosis Executioner Caspase-3" International Journal of Molecular Sciences 17, no. 9: 1427. https://doi.org/10.3390/ijms17091427
APA StyleZhang, N., Zhong, J., Han, S., Li, Y., Yin, Y., & Li, J. (2016). MicroRNA-378 Alleviates Cerebral Ischemic Injury by Negatively Regulating Apoptosis Executioner Caspase-3. International Journal of Molecular Sciences, 17(9), 1427. https://doi.org/10.3390/ijms17091427





