β1 Integrin as a Prognostic and Predictive Marker in Triple-Negative Breast Cancer
Abstract
:1. Introduction
2. Results
2.1. Expression of β1 Integrin in Normal Mammary Epithelial Cells and Triple Negative Breast Cancer (TNBC) Cells
2.2. β1 Integrin Affected TNBC Cell Migration and Invasion
2.3. β1 Integrin Regulated Expressions of Proteins Related to the Epithelial-Mesenchymal Transition (EMT) in TNBC Cells
2.4. Inhibition of β1 Integrin Attenuated Cisplatin-Induced Apoptosis
2.5. β1 Integrin Modulates FAK-Mediated Drug Sensitivity and Cell Survival
2.6. Relationships Between β1 Integrin and Clinicopathological Parameters
2.7. Survival Analysis
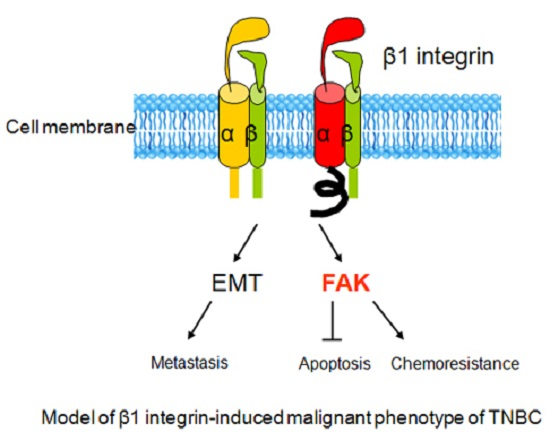
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Reagents
4.3. Immunoblots
4.4. β1 Integrin Short Interfering RNA and FAK Plasmid Transfection
4.5. Cell Proliferation Assay
4.6. Cell Wound Scrape Assay
4.7. Cell Invasion Assay
4.8. Specimens
4.9. Immunohistochemistry (IHC) Staining
4.10. Scoring
4.11. Statistical Analysis
Acknowledgments
Author Contributions
Conflicts of Interest
References
- O'Reilly, E.A.; Gubbins, L.; Sharma, S.; Tully, R.; Guang, M.H.; Weiner-Gorzel, K.; McCaffrey, J.; Harrison, M.; Furlong, F.; Kell, M.; et al. The fate of chemoresistance in triple negative breast cancer (TNBC). BBA Clin. 2015, 3, 257–275. [Google Scholar] [CrossRef] [PubMed]
- Morris, G.J.; Naidu, S.; Topham, A.K.; Guiles, F.; Xu, Y.; McCue, P.; Schwartz, G.F.; Park, P.K.; Rosenberg, A.L.; Brill, K.; et al. Differences in breast carcinoma characteristics in newly diagnosed African-American and Caucasian patients. Cancer 2007, 110, 876–884. [Google Scholar] [CrossRef] [PubMed]
- Haffty, B.G.; Yang, Q.; Reiss, M.; Kearney, T.; Higgins, S.A.; Weidhaas, J.; Harris, L.; Hait, W.; Toppmeyer, D. Locoregional relapse and distant metastasis in conservatively managed triple negative early-stage breast cancer. J. Clin. Oncol. 2006, 24, 5652–5657. [Google Scholar] [CrossRef] [PubMed]
- Viale, G.; Rotmensz, N.; Maisonneuve, P.; Bottiglieri, L.; Montagna, E.; Luini, A.; Veronesi, P.; Intra, M.; Torrisi, R.; Cardillo, A.; et al. Invasive ductal carcinoma of the breast with the “triple-negative” phenotype: Prognostic implications of EGFR immunoreactivity. Breast Cancer Res. Treat. 2009, 116, 317–328. [Google Scholar] [CrossRef] [PubMed]
- Anders, C.K.; Carey, L.A. Biology, metastatic patterns, and treatment of patients with triple-negative breast cancer. Clin. Breast Cancer 2009, 9, S73–S81. [Google Scholar] [CrossRef] [PubMed]
- Bonotto, M.; Gerratana, L.; Poletto, E.; Driol, P.; Giangreco, M.; Russo, S.; Minisini, A.M.; Andreetta, C.; Mansutti, M.; Pisa, F.E.; et al. Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. Oncologist 2014, 19, 608–615. [Google Scholar] [CrossRef] [PubMed]
- Dent, R.; Trudeau, M.; Pritchard, K.I.; Hanna, W.M.; Kahn, H.K.; Sawka, C.A.; Lickley, L.A.; Rawlinson, E.; Sun, P.; Narod, S.A. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin. Cancer Res. 2007, 13, 4429–4434. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, H.; Wyckoff, J.; Condeelis, J. Cell migration in tumors. Curr. Opin. Cell Biol. 2005, 17, 559–564. [Google Scholar] [CrossRef] [PubMed]
- Gilcrease, M.Z. Integrin signaling in epithelial cells. Cancer Lett. 2007, 247, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Esposito, M.; Kang, Y. Targeting tumor-stromal interactions in bone metastasis. Pharmacol. Ther. 2014, 141, 222–233. [Google Scholar] [CrossRef] [PubMed]
- Naci, D.; Vuori, K.; Aoudjit, F. α2β1 integrin in cancer development and chemoresistance. Semin. Cancer Biol. 2015, 35, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Scales, T.M.; Parsons, M. Spatial and temporal regulation of integrin signalling during cell migration. Curr. Opin. Cell Biol. 2011, 23, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos, P.B.; Zanetti, J.S.; Ribeiro-Silva, A.; Beltrao, E.I. β1 integrin predicts survival in breast cancer: A clinicopathological and immunohistochemical study. Diagn. Pathol. 2012, 7, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blandin, A.F.; Renner, G.; Lehmann, M.; Lelong-Rebel, I.; Martin, S.; Dontenwill, M. β1 Integrins as therapeutic targets to disrupt hallmarks of cancer. Front. Pharmacol. 2015, 6, 279. [Google Scholar] [CrossRef]
- Kenny, P.A.; Lee, G.Y.; Myers, C.A.; Neve, R.M.; Semeiks, J.R.; Spellman, P.T.; Lorenz, K.; Lee, E.H.; Barcellos-Hoff, M.H.; Petersen, O.W.; et al. The morphologies of breast cancer cell lines in three-dimensional assays correlate with their profiles of gene expression. Mol. Oncol. 2007, 1, 84–96. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.J.; Zhu, Z.X.; Zhou, J.S.; Hu, Z.Q.; Zhang, J.P.; Cai, Q.P.; Wang, L.H. Silencing profilin-1 inhibits gastric cancer progression via integrin β1/focal adhesion kinase pathway modulation. World J. Gastroenterol. 2015, 21, 2323–2335. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Gong, K.; Wohlfeld, B.; Hatanpaa, K.J.; Zhao, D.; Habib, A.A. Ligand-Independent EGFR Signaling. Cancer Res. 2015, 75, 3436–3441. [Google Scholar] [CrossRef] [PubMed]
- Hu, C.; Ni, Z.; Li, B.S.; Yong, X.; Yang, X.; Zhang, J.W.; Zhang, D.; Qin, Y.; Jie, M.M.; Dong, H.; et al. hTERT promotes the invasion of gastric cancer cells by enhancing FOXO3a ubiquitination and subsequent ITGB1 upregulation. Gut 2015. [Google Scholar] [CrossRef]
- Li, N.; Zhang, Y.; Naylor, M.J.; Schatzmann, F.; Maurer, F.; Wintermantel, T.; Schuetz, G.; Mueller, U.; Streuli, C.H.; Hynes, N.E. β1 integrins regulate mammary gland proliferation and maintain the integrity of mammary alveoli. EMBO J. 2005, 24, 1942–1953. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ishihara, S.; Yasuda, M.; Nishioka, T.; Mizutani, T.; Ishikawa, M.; Kawabata, K.; Shirato, H.; Haga, H. Lung cancer cells that survive ionizing radiation show increased integrin α2β1- and EGFR-dependent invasiveness. PLoS ONE 2013, 8, e70905. [Google Scholar] [CrossRef] [PubMed]
- Mai, A.; Muharram, G.; Barrow-McGee, R.; Baghirov, H.; Rantala, J.; Kermorgant, S.; Ivaska, J. Distinct c-Met activation mechanisms induce cell rounding or invasion through pathways involving integrins, RhoA and HIP1. J. Cell Sci. 2014, 127, 1938–1952. [Google Scholar] [CrossRef] [PubMed]
- Williams, K.C.; Coppolino, M.G. SNARE-dependent interaction of Src, EGFR and β1 integrin regulates invadopodia formation and tumor cell invasion. J. Cell Sci. 2014, 127, 1712–1725. [Google Scholar] [CrossRef] [PubMed]
- Zhou, P.; Erfani, S.; Liu, Z.; Jia, C.; Chen, Y.; Xu, B.; Deng, X.; Alfaro, J.E.; Chen, L.; Napier, D.; et al. CD151-α3β1 integrin complexes are prognostic markers of glioblastoma and cooperate with EGFR to drive tumor cell motility and invasion. Oncotarget 2015, 6, 29675–29693. [Google Scholar] [PubMed]
- Fedorenko, I.V.; Abel, E.V.; Koomen, J.M.; Fang, B.; Wood, E.R.; Chen, Y.A.; Fisher, K.J.; Iyengar, S.; Dahlman, K.B.; Wargo, J.A.; et al. Fibronectin induction abrogates the BRAF inhibitor response of BRAF V600E/PTEN-null melanoma cells. Oncogene 2016, 35, 1225–1235. [Google Scholar] [CrossRef] [PubMed]
- Hirata, E.; Girotti, M.R.; Viros, A.; Hooper, S.; Spencer-Dene, B.; Matsuda, M.; Larkin, J.; Marais, R.; Sahai, E. Intravital imaging reveals how BRAF inhibition generates drug-tolerant microenvironments with high integrin β1/FAK signaling. Cancer Cell 2015, 27, 574–588. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Park, C.C.; Hilsenbeck, S.G.; Ward, R.; Rimawi, M.F.; Wang, Y.C.; Shou, J.; Bissell, M.J.; Osborne, C.K.; Schiff, R. β1 integrin mediates an alternative survival pathway in breast cancer cells resistant to lapatinib. Breast Cancer Res. 2011, 13, R84. [Google Scholar] [CrossRef] [PubMed]
- McGrail, D.J.; Khambhati, N.N.; Qi, M.X.; Patel, K.S.; Ravikumar, N.; Brandenburg, C.P.; Dawson, M.R. Alterations in ovarian cancer cell adhesion drive taxol resistance by increasing microtubule dynamics in a FAK-dependent manner. Sci. Rep. 2015, 5, 9529. [Google Scholar] [CrossRef] [PubMed]
- Yuan, J.; Liu, M.; Yang, L.; Tu, G.; Zhu, Q.; Chen, M.; Cheng, H.; Luo, H.; Fu, W.; Li, Z.; et al. Acquisition of epithelial-mesenchymal transition phenotype in the tamoxifen-resistant breast cancer cell: A new role for G protein-coupled estrogen receptor in mediating tamoxifen resistance through cancer-associated fibroblast-derived fibronectin and β1-integrin signaling pathway in tumor cells. Breast Cancer Res. 2015, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Bhaskar, V.; Zhang, D.; Fox, M.; Seto, P.; Wong, M.H.; Wales, P.E.; Powers, D.; Chao, D.T.; Dubridge, R.B.; Ramakrishnan, V. A function blocking anti-mouse integrin α5β1 antibody inhibits angiogenesis and impedes tumor growth in vivo. J. Transl. Med. 2007, 5, 61. [Google Scholar] [CrossRef] [PubMed]
- Ricart, A.D.; Tolcher, A.W.; Liu, G.; Holen, K.; Schwartz, G.; Albertini, M.; Weiss, G.; Yazji, S.; Ng, C.; Wilding, G. Volociximab, a chimeric monoclonal antibody that specifically binds α5β1 integrin: A phase I, pharmacokinetic, and biological correlative study. Clin. Cancer Res. 2008, 14, 7924–7929. [Google Scholar] [CrossRef] [PubMed]
- Park, C.C.; Zhang, H.J.; Yao, E.S.; Park, C.J.; Bissell, M.J. β1 integrin inhibition dramatically enhances radiotherapy efficacy in human breast cancer xenografts. Cancer Res. 2008, 68, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Desgrosellier, J.S.; Cheresh, D.A. Integrins in cancer: Biological implications and therapeutic opportunities. Nat. Rev. Cancer 2010, 10, 9–22. [Google Scholar] [CrossRef] [PubMed]
- Luo, C.W.; Wu, C.C.; Ch’ang, H.J. Radiation sensitization of tumor cells induced by shear stress: The roles of integrins and FAK. Biochim. Biophys. Acta 2014, 1843, 2129–2137. [Google Scholar] [CrossRef] [PubMed]
- Ju, L.; Zhou, C. Integrin β1 enhances the epithelial-mesenchymal transition in association with gefitinib resistance of non-small cell lung cancer. Cancer Biomark. 2013, 13, 329–336. [Google Scholar] [PubMed]
- Mantoni, T.S.; Lunardi, S.; Al-Assar, O.; Masamune, A.; Brunner, T.B. Pancreatic stellate cells radioprotect pancreatic cancer cells through β1-integrin signaling. Cancer Res. 2011, 71, 3453–3458. [Google Scholar] [CrossRef] [PubMed]
- Burkhalter, R.J.; Symowicz, J.; Hudson, L.G.; Gottardi, C.J.; Stack, M.S. Integrin regulation of β-catenin signaling in ovarian carcinoma. J. Biol. Chem. 2011, 286, 23467–23475. [Google Scholar] [CrossRef] [PubMed]
- Barkan, D.; Chambers, A.F. β1-integrin: A potential therapeutic target in the battle against cancer recurrence. Clin. Cancer Res. 2011, 17, 7219–7223. [Google Scholar] [CrossRef] [PubMed]
- Gerratana, L.; Fanotto, V.; Pelizzari, G.; Agostinetto, E.; Puglisi, F. Do platinum salts fit all triple negative breast cancers? Cancer Treat. Rev. 2016, 48, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Yao, E.S.; Zhang, H.; Chen, Y.Y.; Lee, B.; Chew, K.; Moore, D.; Park, C. Increased β1 integrin is associated with decreased survival in invasive breast cancer. Cancer Res. 2007, 67, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Klahan, S.; Huang, W.C.; Chang, C.M.; Wong, H.S.; Huang, C.C.; Wu, M.S.; Lin, Y.C.; Lu, H.F.; Hou, M.F.; Chang, W.C. Gene expression profiling combined with functional analysis identify integrin β1 (ITGB1) as a potential prognosis biomarker in triple negative breast cancer. Pharmacol. Res. 2016, 104, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Bulfoni, M.; Gerratana, L.; del Ben, F.; Marzinotto, S.; Sorrentino, M.; Turetta, M.; Scoles, G.; Toffoletto, B.; Isola, M.; Beltrami, C.A.; et al. In patients with metastatic breast cancer the identification of circulating tumor cells in epithelial-to-mesenchymal transition is associated with a poor prognosis. Breast Cancer Res. 2016, 18, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, R.M.; Chin, K.; Fridlyand, J.; Yeh, J.; Baehner, F.L.; Fevr, T.; Clark, L.; Bayani, N.; Coppe, J.P.; Tong, F.; et al. A collection of breast cancer cell lines for the study of functionally distinct cancer subtypes. Cancer Cell 2006, 10, 515–527. [Google Scholar] [CrossRef] [PubMed]
- Zajchowski, D.A.; Bartholdi, M.F.; Gong, Y.; Webster, L.; Liu, H.L.; Munishkin, A.; Beauheim, C.; Harvey, S.; Ethier, S.P.; Johnson, P.H. Identification of gene expression profiles that predict the aggressive behavior of breast cancer cells. Cancer Res. 2001, 61, 5168–5178. [Google Scholar] [PubMed]
- Aoudjit, F.; Vuori, K. Integrin signaling inhibits paclitaxel-induced apoptosis in breast cancer cells. Oncogene 2001, 20, 4995–5004. [Google Scholar] [CrossRef] [PubMed]
- Damiano, J.S.; Cress, A.E.; Hazlehurst, L.A.; Shtil, A.A.; Dalton, W.S. Cell adhesion mediated drug resistance (CAM-DR): Role of integrins and resistance to apoptosis in human myeloma cell lines. Blood 1999, 93, 1658–1667. [Google Scholar] [PubMed]
- Hodkinson, P.S.; Mackinnon, A.C.; Sethi, T. Extracellular matrix regulation of drug resistance in small-cell lung cancer. Int. J. Radiat. Biol. 2007, 83, 733–741. [Google Scholar] [CrossRef] [PubMed]
- Jahangiri, A.; Aghi, M.K.; Carbonell, W.S. β1 integrin: Critical path to antiangiogenic therapy resistance and beyond. Cancer Res. 2014, 74, 3–7. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Enomoto, A.; Watanabe, T.; Haga, H.; Ishida, S.; Kondo, Y.; Furukawa, K.; Urano, T.; Mii, S.; Weng, L.; et al. TRIM27/MRTF-B-dependent integrin β1 expression defines leading cells in cancer cell collectives. Cell Rep. 2014, 7, 1156–1167. [Google Scholar] [CrossRef] [PubMed]
- Kurozumi, A.; Goto, Y.; Matsushita, R.; Fukumoto, I.; Kato, M.; Nishikawa, R.; Sakamoto, S.; Enokida, H.; Nakagawa, M.; Ichikawa, T.; et al. Tumor-suppressive microRNA-223 inhibits cancer cell migration and invasion by targeting ITGA3/ITGB1 signaling in prostate cancer. Cancer Sci. 2016, 107, 84–94. [Google Scholar] [CrossRef] [PubMed]






| Protein Expression | β1 Integrin, n (%) | p-Value | ||
|---|---|---|---|---|
| High | Low | |||
| High | 22 (32.83) | 19 (28.36) | ||
| FAK, n (%) | ||||
| Low | 7 (10.45) | 19 (28.36) | p = 0.031 | |
| Parameters | n | β1 Integrin, n (%) | p-Value | |
|---|---|---|---|---|
| Low | High | |||
| Total | 67 | 38 (56.72) | 29 (43.28) | |
| Age, n (%) | 0.0878 | |||
| ≤40 years | 57 | 35 (92.11) | 22 (75.86) | |
| >40 years | 10 | 3 (7.89) | 7 (24.14) | |
| Size, n (%) | 0.4753 | |||
| ≤2.0 cm | 24 | 15 (39.47) | 9 (31.03) | |
| >2.0 cm | 43 | 23 (60.53) | 20 (68.97) | |
| Grade, n (%) | 0.7530 | |||
| I/II | 24 | 13 (34.21) | 11 (37.93) | |
| III | 43 | 25 (65.79) | 18 (62.07) | |
| Tumor stage, n (%) | 0.2893 | |||
| T1 | 28 | 18 (47.37) | 10 (34.48) | |
| T2/T3 | 39 | 20 (52.63) | 19 (65.52) | |
| Nodal stage, n (%) | 0.0958 | |||
| N0 | 40 | 26 (68.42) | 14 (48.28) | |
| N1/N2/N3 | 27 | 12 (31.58) | 15 (51.72) | |
| Metastatic stage, n (%) | 0.0389 * | |||
| M0 | 48 | 31 (81.58) | 17 (58.62) | |
| M1 | 19 | 7 (18.42) | 12 (41.38) | |
| Tumor recurrent, n (%) | 0.0380 * | |||
| Absent | 61 | 37 (97.37) | 24 (82.76) | |
| Present | 6 | 1 (2.63) | 5 (17.24) | |
| Survival status, n (%) | 0.0040 * | |||
| Survival | 45 | 31 (81.58) | 14 (48.28) | |
| Death | 22 | 7 (18.42) | 15 (51.72) | |
| Factors | Univariate | Multivariate | ||
|---|---|---|---|---|
| HR (95% CI) | p-Value | HR (95% CI) | p-Value | |
| β1 integrin expression | 0.0040 * | 0.0476 * | ||
| Low | 1.0 | 1.0 | ||
| High | 3.805 (1.533–9.446) | 2.772 (1.011–7.600) | ||
| Age | 0.8954 | 0.9776 | ||
| ≤40 years | 1.0 | 1.0 | ||
| >40 years | 0.921 (0.269–3.149) | 0.981 (0.254–3.784) | ||
| Grade | 0.1240 | 0.1565 | ||
| I/II | 1.0 | 1.0 | ||
| III | 2.190 (0.807–5.947) | 2.286 (0.728–7.172) | ||
| Nodal stage | <0.0001 * | 0.0005 * | ||
| N0 | 1.0 | 1.0 | ||
| N1/N2/N3 | 12.304(3.634–41.656) | 9.602 (2.666–34.586) | ||
| Metastatic stage | 0.0019 * | 0.4788 | ||
| M0 | 1.0 | 1.0 | ||
| M1 | 3.895 (1.653–9.178) | 1.462 (0.511–4.186) | ||
| Tumor recurrent | 0.0436 * | 0.8340 | ||
| Absent | 1.0 | 1.0 | ||
| Present | 3.077 (1.033–9.165) | 1.142 (0.329–3.969) | ||
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yin, H.-L.; Wu, C.-C.; Lin, C.-H.; Chai, C.-Y.; Hou, M.-F.; Chang, S.-J.; Tsai, H.-P.; Hung, W.-C.; Pan, M.-R.; Luo, C.-W. β1 Integrin as a Prognostic and Predictive Marker in Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2016, 17, 1432. https://doi.org/10.3390/ijms17091432
Yin H-L, Wu C-C, Lin C-H, Chai C-Y, Hou M-F, Chang S-J, Tsai H-P, Hung W-C, Pan M-R, Luo C-W. β1 Integrin as a Prognostic and Predictive Marker in Triple-Negative Breast Cancer. International Journal of Molecular Sciences. 2016; 17(9):1432. https://doi.org/10.3390/ijms17091432
Chicago/Turabian StyleYin, Hsin-Ling, Chun-Chieh Wu, Chih-Hung Lin, Chee-Yin Chai, Ming-Feng Hou, Shu-Jyuan Chang, Hung-Pei Tsai, Wen-Chun Hung, Mei-Ren Pan, and Chi-Wen Luo. 2016. "β1 Integrin as a Prognostic and Predictive Marker in Triple-Negative Breast Cancer" International Journal of Molecular Sciences 17, no. 9: 1432. https://doi.org/10.3390/ijms17091432