Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster
Abstract
:1. Introduction
2. Results
2.1. Polydeoxyribonucleotide and Placentex® Inhibit Melanogenesis in Mel-Ab Cells and in Human Melanocyte–Keratinocyte Cocultures
2.2. Polydeoxyribonucleotide and Placentex® Suppress Intracellular Tyrosinase Activity in Mel-Ab Cells
2.3. Polydeoxyribonucleotide and Placentex® Reduce the Levels of Microphthalmia-Associated Transcription Factor and Melanogenesis-Related Proteins
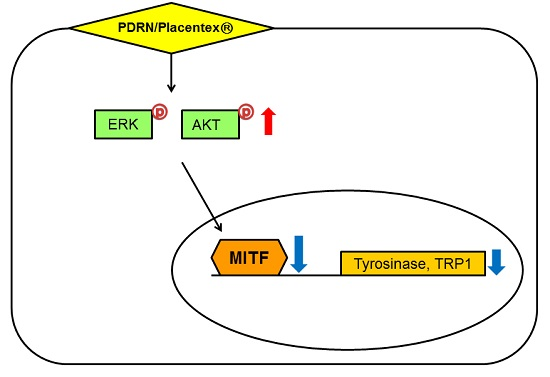
2.4. Polydeoxyribonucleotide (PDRN) Affects the Levels of Melanogenesis-Related Signaling Pathways
2.5. Clinical Efficacy of Placentex® for the Treatment of Hyperpigmentation
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture
4.3. Measurement of Melanin Content
4.4. Intracellular Tyrosinase Activity Assay
4.5. Western Immunoblotting
4.6. Patients and Study Design
4.7. Statistical Analysis
Author Contributions
Conflicts of Interest
Abbreviations
| AMP | adenosine monophosphate |
| ANOVA | analysis of variance |
| CT | Cholera toxin |
| DMEM | Dulbecco’s modified Eagle’s medium |
| DUSP | Dual specific phosphatase |
| EGF | Epidermal growth factor |
| ERK | Extracellular signal-regulated protein kinase |
| FBS | Fetal bovine serum |
| GSK3β | Glycogen synthase kinase 3β |
| HKGS | Human keratinocyte growth supplement |
| JNK | C-Jun N-terminal kinase |
| l-DOPA | l-3,4-dihydroxyphenylalanine |
| LPS | Lipopolysaccharide |
| MAP | Mitogen-activated protein |
| MITF | Microphthalmia-associated transcription factor |
| OD | Optical density |
| PBS | Phosphate-buffered saline |
| PDRN | Polydeoxyribonucleotide |
| PG | prostaglandin |
| PIH | Post-inflammatory hyperpigmentation |
| PI3K | Phosphatidylinositide 3-kinase |
| PMP | Photoaging-associated mottled pigmentation |
| PTU | N’-phenylthiourea |
| TNF | Tumor necrosis factor |
| TPA | Phorbol 12-myristate 13-acetate |
| TRP | Tyrosinase-related protein |
| UV | Ultraviolet |
| VEGF | Vascular endothelial growth factor |
References
- Lee, S.H.; Zheng, Z.; Kang, J.S.; Kim, D.Y.; Oh, S.H.; Cho, S.B. Therapeutic efficacy of autologous platelet-rich plasma and polydeoxyribonucleotide on female pattern hair loss. Wound Repair Regen. 2015, 23, 30–36. [Google Scholar] [CrossRef] [PubMed]
- Squadrito, F.; Bitto, A.; Altavilla, D.; Arcoraci, V.; de Caridi, G.; de Feo, M.E.; Corrao, S.; Pallio, G.; Sterrantino, C.; Minutoli, L.; et al. The effect of PDRN, an adenosine receptor A2A agonist, on the healing of chronic diabetic foot ulcers: Results of a clinical trial. J. Clin. Endocrinol. Metab. 2014, 99, E746–E753. [Google Scholar] [CrossRef] [PubMed]
- Galeano, M.; Bitto, A.; Altavilla, D.; Minutoli, L.; Polito, F.; Calo, M.; Lo Cascio, P.; Stagno d'Alcontres, F.; Squadrito, F. Polydeoxyribonucleotide stimulates angiogenesis and wound healing in the genetically diabetic mouse. Wound Repair Regen. 2008, 16, 208–217. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Polito, F.; Irrera, N.; D'Ascola, A.; Avenoso, A.; Nastasi, G.; Campo, G.M.; Micali, A.; Bagnato, G.; Minutoli, L.; et al. Polydeoxyribonucleotide reduces cytokine production and the severity of collagen-induced arthritis by stimulation of adenosine A2A receptor. Arthritis Rheum. 2011, 63, 3364–3371. [Google Scholar] [CrossRef] [PubMed]
- Koroskenyi, K.; Kiss, B.; Szondy, Z. Adenosine A2A receptor signaling attenuates LPS-induced pro-inflammatory cytokine formation of mouse macrophages by inducing the expression of DUSP1. Biochim. Biophys. Acta 2016, 1863, 1461–1471. [Google Scholar] [CrossRef] [PubMed]
- Bitto, A.; Oteri, G.; Pisano, M.; Polito, F.; Irrera, N.; Minutoli, L.; Squadrito, F.; Altavilla, D. Adenosine receptor stimulation by polynucleotides (PDRN) reduces inflammation in experimental periodontitis. J. Clin. Periodontol. 2013, 40, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.Y.; Lee, H.E.; Im, M.; Lee, Y.; Kim, C.D.; Lee, J.H.; Seo, Y.J. Effect of adenosine on melanogenesis in B16 cells and zebrafish. Ann. Dermatol. 2014, 26, 209–213. [Google Scholar] [CrossRef] [PubMed]
- Yun, W.J.; Bang, S.H.; Min, K.H.; Kim, S.W.; Lee, M.W.; Chang, S.E. Epidermal growth factor and epidermal growth factor signaling attenuate laser-induced melanogenesis. Dermatol. Surg. 2013, 39, 1903–1911. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Lee, W.J.; Chang, S.E.; Lee, G.Y. Hesperidin, A Popular Antioxidant Inhibits Melanogenesis via ERK1/2 Mediated MITF Degradation. Int. J. Mol. Sci. 2015, 16, 18384–18395. [Google Scholar] [CrossRef] [PubMed]
- Chung, B.Y.; Kim, S.Y.; Jung, J.M.; Won, C.H.; Choi, J.H.; Lee, M.W.; Chang, S.E. The antimycotic agent clotrimazole inhibits melanogenesis by accelerating ERK and PI3K-/AKT-mediated tyrosinase degradation. Exp. Dermatol. 2015, 24, 386–388. [Google Scholar] [CrossRef] [PubMed]
- Kameyama, K.; Sakai, C.; Kuge, S.; Nishiyama, S.; Tomita, Y.; Ito, S.; Wakamatsu, K.; Hearing, V.J. The expression of tyrosinase, tyrosinase-related proteins 1 and 2 (TRP1 and TRP2), the silver protein, and a melanogenic inhibitor in human melanoma cells of differing melanogenic activities. Pigment. Cell Res. 1995, 8, 97–104. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.J.; Jo, S.Y.; Lee, M.H.; Won, C.H.; Lee, M.W.; Choi, J.H.; Chang, S.E. The Effect of MCP-1/CCR2 on the Proliferation and Senescence of Epidermal Constituent Cells in Solar Lentigo. Int. J. Mol. Sci. 2016, 17, 948. [Google Scholar] [CrossRef] [PubMed]
- Prota, G. Regulatory mechanisms of melanogenesis: beyond the tyrosinase concept. J. Investig. Dermatol. 1993, 100, 156s–161s. [Google Scholar] [PubMed]
- Shibahara, S.; Yasumoto, K.; Amae, S.; Udono, T.; Watanabe, K.; Saito, H.; Takeda, K. Regulation of pigment cell-specific gene expression by MITF. Pigment. Cell Res. 2000, 13 (Suppl. 8), 98–102. [Google Scholar] [CrossRef] [PubMed]
- Sini, P.; Denti, A.; Cattarini, G.; Daglio, M.; Tira, M.E.; Balduini, C. Effect of polydeoxyribonucleotides on human fibroblasts in primary culture. Cell Biochem. Funct. 1999, 17, 107–114. [Google Scholar] [CrossRef] [PubMed]
- Tonello, G.; Daglio, M.; Zaccarelli, N.; Sottofattori, E.; Mazzei, M.; Balbi, A. Characterization and quantitation of the active polynucleotide fraction (PDRN) from human placenta, a tissue repair stimulating agent. J. Pharm. Biomed. Anal. 1996, 14, 1555–1560. [Google Scholar] [CrossRef] [PubMed]
- Belletti, S.; Uggeri, J.; Gatti, R.; Govoni, P.; Guizzardi, S. Polydeoxyribonucleotide promotes cyclobutane pyrimidine dimer repair in UVB-exposed dermal fibroblasts. Photodermatol. Photoimmunol. Photomed. 2007, 23, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Rathbone, M.P.; Christjanson, L.; Deforge, S.; Deluca, B.; Gysbers, J.W.; Hindley, S.; Jovetich, M.; Middlemiss, P.; Takhal, S. Extracellular purine nucleosides stimulate cell division and morphogenesis: Pathological and physiological implications. Med. Hypotheses 1992, 37, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.A.; Gao, Z.G. Adenosine receptors as therapeutic targets. Nat. Rev. Drug Discov. 2006, 5, 247–264. [Google Scholar] [CrossRef] [PubMed]
- Cronstein, B.N. Adenosine, an endogenous anti-inflammatory agent. J. Appl. Physiol. 1994, 76, 5–13. [Google Scholar] [PubMed]
- Streitova, D.; Hofer, M.; Hola, J.; Vacek, A.; Pospisil, M. Adenosine A1, A2a, A2b, and A3 receptors in hematopoiesis. 2. Expression of receptor mRNA in resting and lipopolysaccharide-activated mouse RAW 264.7 macrophages. Physiol. Res. 2010, 59, 139–144. [Google Scholar] [PubMed]
- Guizzardi, S.; Galli, C.; Govoni, P.; Boratto, R.; Cattarini, G.; Martini, D.; Belletti, S.; Scandroglio, R. Polydeoxyribonucleotide (PDRN) promotes human osteoblast proliferation: A new proposal for bone tissue repair. Life Sci. 2003, 73, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Montesinos, M.C.; Gadangi, P.; Longaker, M.; Sung, J.; Levine, J.; Nilsen, D.; Reibman, J.; Li, M.; Jiang, C.K.; Hirschhorn, R.; et al. Wound healing is accelerated by agonists of adenosine A2 (Gαs-linked) receptors. J. Exp. Med. 1997, 186, 1615–1620. [Google Scholar] [CrossRef] [PubMed]
- Nayak, S.; Khan, M.A.; Wan, T.C.; Pei, H.; Linden, J.; Dwinell, M.R.; Geurts, A.M.; Imig, J.D.; Auchampach, J.A. Characterization of Dahl salt-sensitive rats with genetic disruption of the A2B adenosine receptor gene: Implications for A2B adenosine receptor signaling during hypertension. Purinergic Signal 2015, 11, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Busca, R.; Bertolotto, C.; Ortonne, J.P.; Ballotti, R. Inhibition of the phosphatidylinositol 3-Kinase/p70S6-kinase pathway induces B16 melanoma cell differentiation. J. Biol. Chem. 1996, 271, 31824–31830. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.J.; Park, J.I.; Lee, J.E.; Myung, C.H.; Kim, S.Y.; Chang, S.E.; Hwang, J.S. A Novel Role of Serotonin Receptor 2B Agonist as an Anti-Melanogenesis Agent. Int. J. Mol. Sci. 2016, 17, 546. [Google Scholar] [CrossRef] [PubMed]
- Jung, J.M.; Kim, S.Y.; Lee, W.J.; Hwang, J.S.; Chang, S.E. Dopamine D4 receptor antagonist inhibits melanogenesis through transcriptional downregulation of MITF via ERK signalling. Exp. Dermatol. 2016, 25, 325–328. [Google Scholar] [CrossRef] [PubMed]
- Arena, S.; Minutoli, L.; Arena, F.; Nicotina, P.A.; Romeo, C.; Squadrito, F.; Altavilla, D.; Morgia, G.; Magno, C. Polydeoxyribonucleotide administration improves the intra-testicular vascularization in rat experimental varicocele. Fertil. Steril. 2012, 97, 165–168. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Shang, J.; Song, J.; Ping, F. Interleukin-18 augments growth ability of primary human melanocytes by PTEN inactivation through the AKT/NF-κB pathway. Int. J. Biochem. Cell Biol. 2013, 45, 308–316. [Google Scholar] [CrossRef] [PubMed]





| Patient Number | Sex | Age (Years) | Diagnosis | Area | Improvement Score |
|---|---|---|---|---|---|
| 1 | F | 36 | Melasma | Periocular | 4 |
| 2 | F | 43 | Melasma | Periocular | 3 |
| 3 | F | 52 | Melasma | Malar, cheek | 5 |
| 4 | F | 34 | PMP | Cheek | 2 |
| 5 | F | 40 | PMP | Cheek | 2 |
| 6 | F | 66 | Pigmented contact dermatitis | Face and neck | 5 |
| mean | 45.2 | - | - | 3.7 | |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noh, T.K.; Chung, B.Y.; Kim, S.Y.; Lee, M.H.; Kim, M.J.; Youn, C.S.; Lee, M.W.; Chang, S.E. Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster. Int. J. Mol. Sci. 2016, 17, 1448. https://doi.org/10.3390/ijms17091448
Noh TK, Chung BY, Kim SY, Lee MH, Kim MJ, Youn CS, Lee MW, Chang SE. Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster. International Journal of Molecular Sciences. 2016; 17(9):1448. https://doi.org/10.3390/ijms17091448
Chicago/Turabian StyleNoh, Tai Kyung, Bo Young Chung, Su Yeon Kim, Mi Hye Lee, Moon Jung Kim, Choon Shik Youn, Mi Woo Lee, and Sung Eun Chang. 2016. "Novel Anti-Melanogenesis Properties of Polydeoxyribonucleotide, a Popular Wound Healing Booster" International Journal of Molecular Sciences 17, no. 9: 1448. https://doi.org/10.3390/ijms17091448