Multiple Activities of Punica granatum Linne against Acne Vulgaris
Abstract
:1. Introduction
2. Results and Discussion
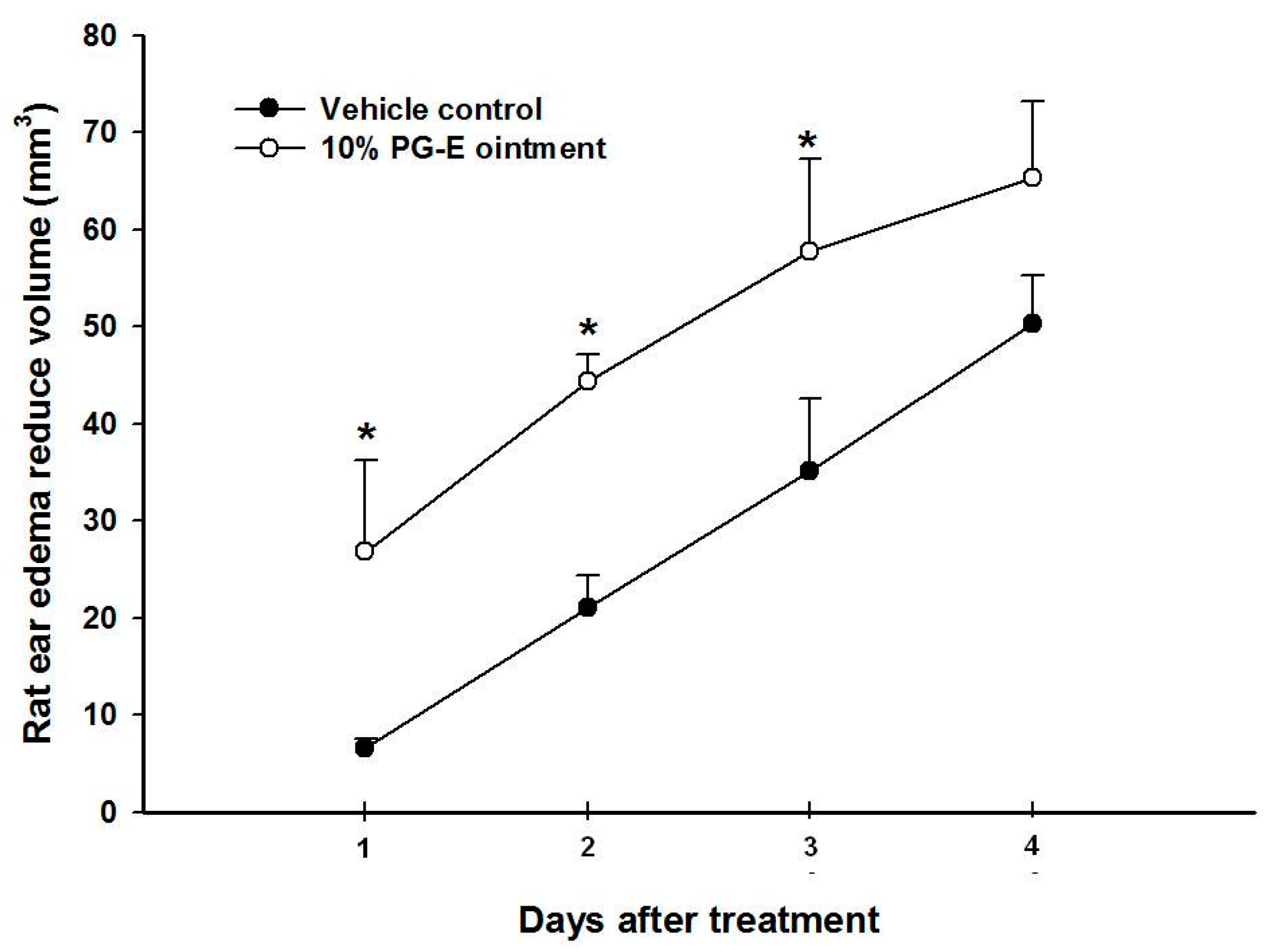
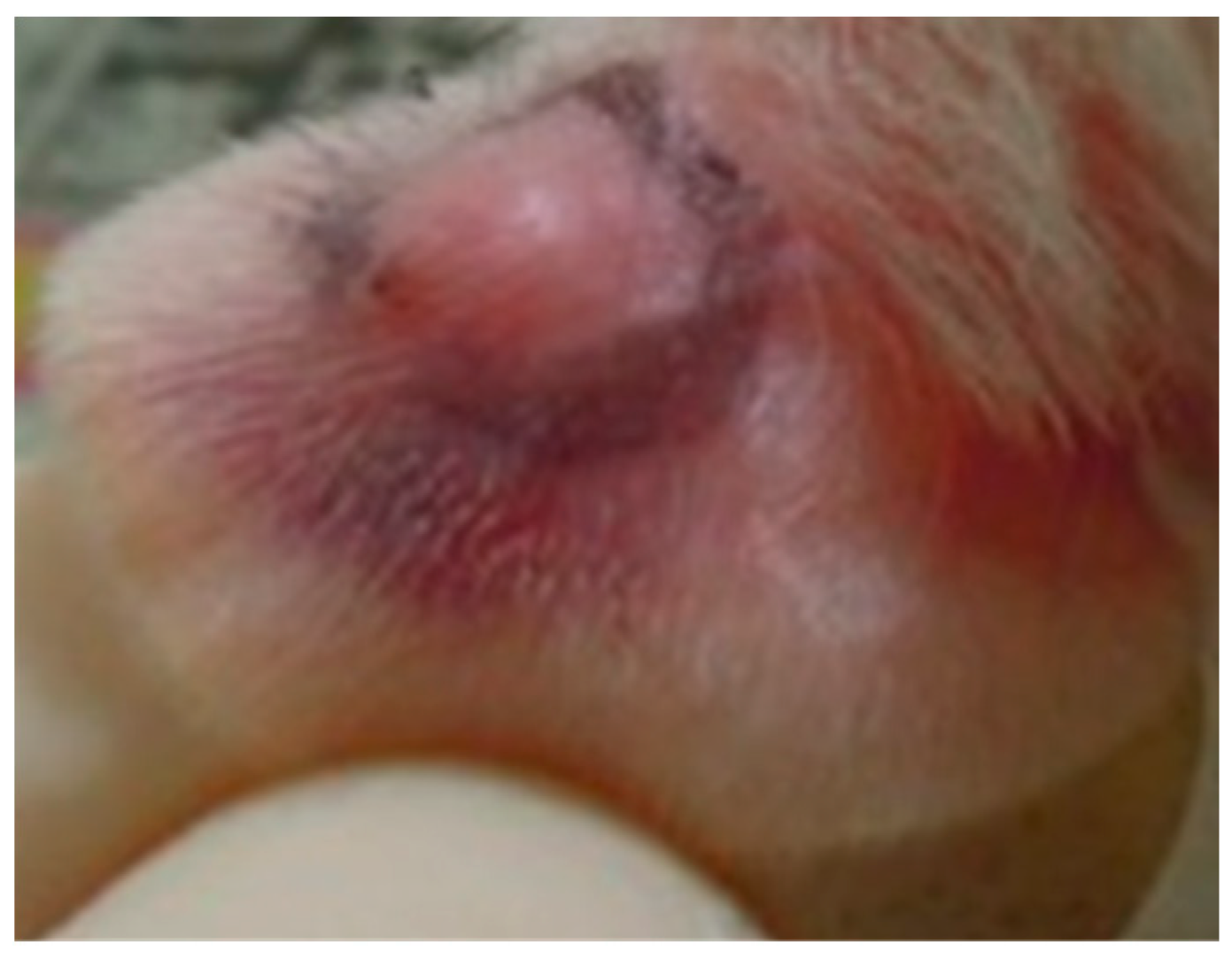
2.1. Pomegranate Attenuate P. acnes-Induced Wistar Ear Edema
2.2. Pomegranate Displayed No Skin Irritation
2.3. Pomegranate Significantly Inhibited P. acnes and S. aureus Growth
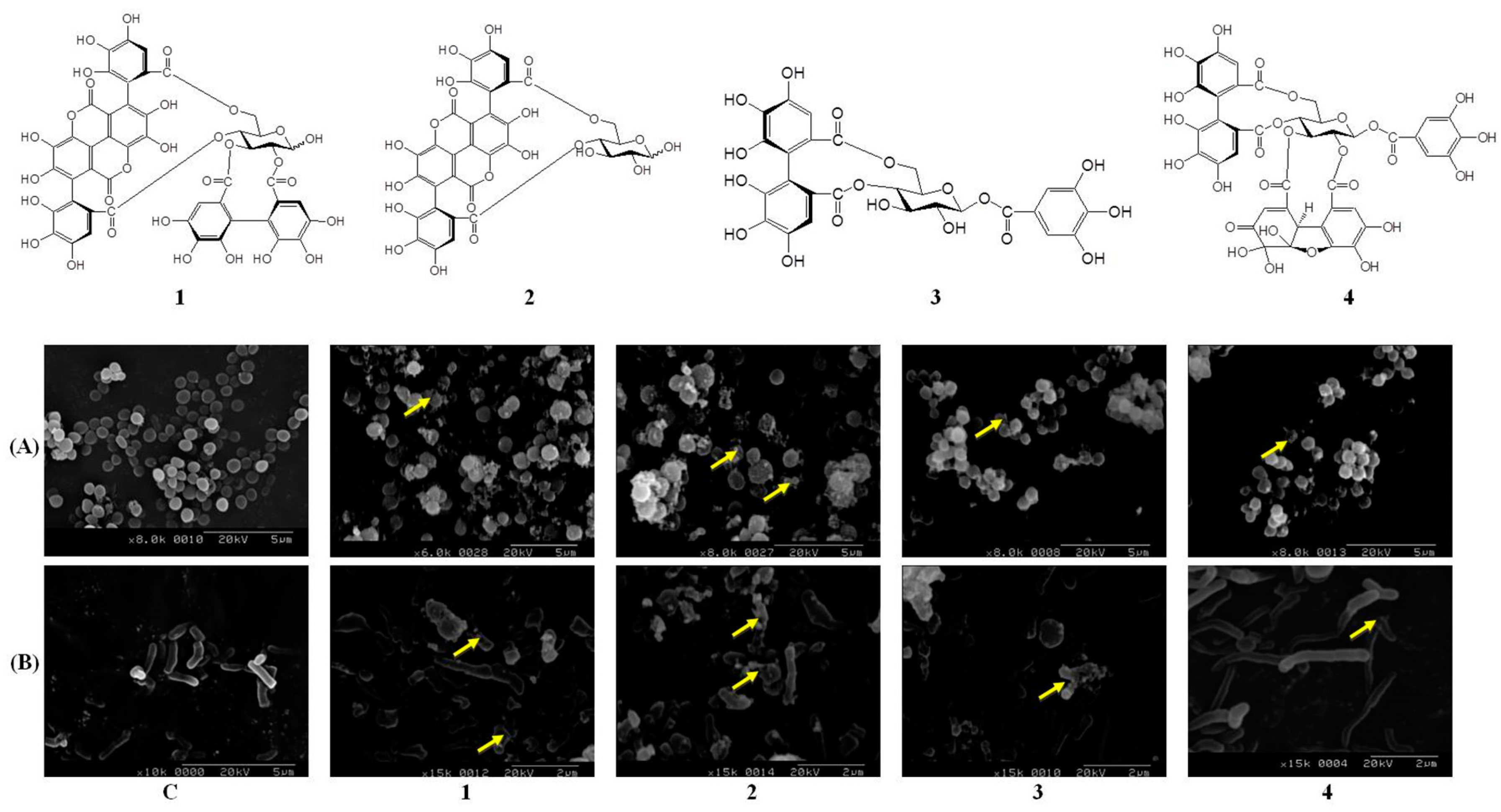
2.4. Pomegranate Polyphenols Caused Shrinkage and Damage in P. acnes and S. aureus
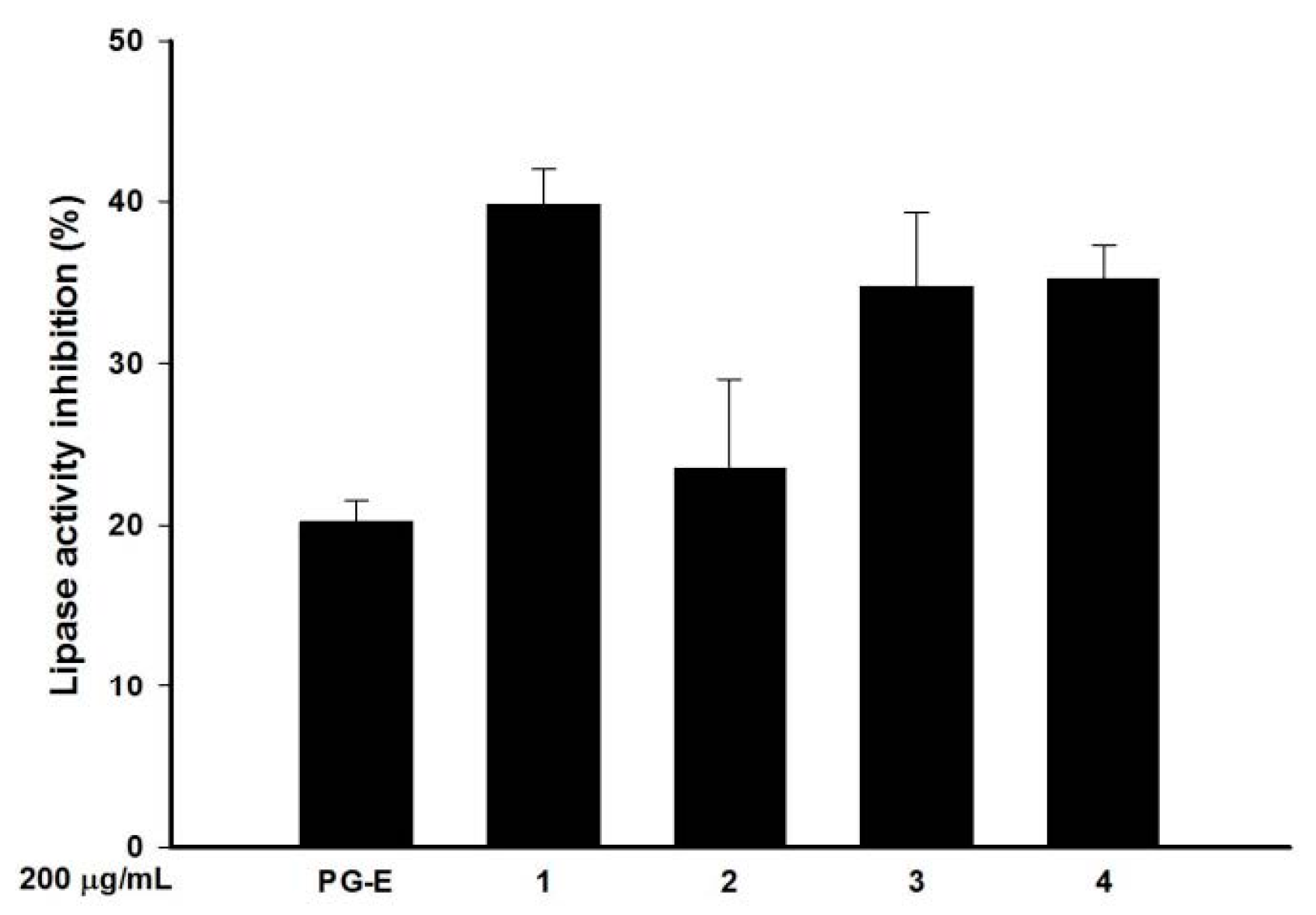
2.5. Pomegranate Polyphenols Inhibited Lipase Activity
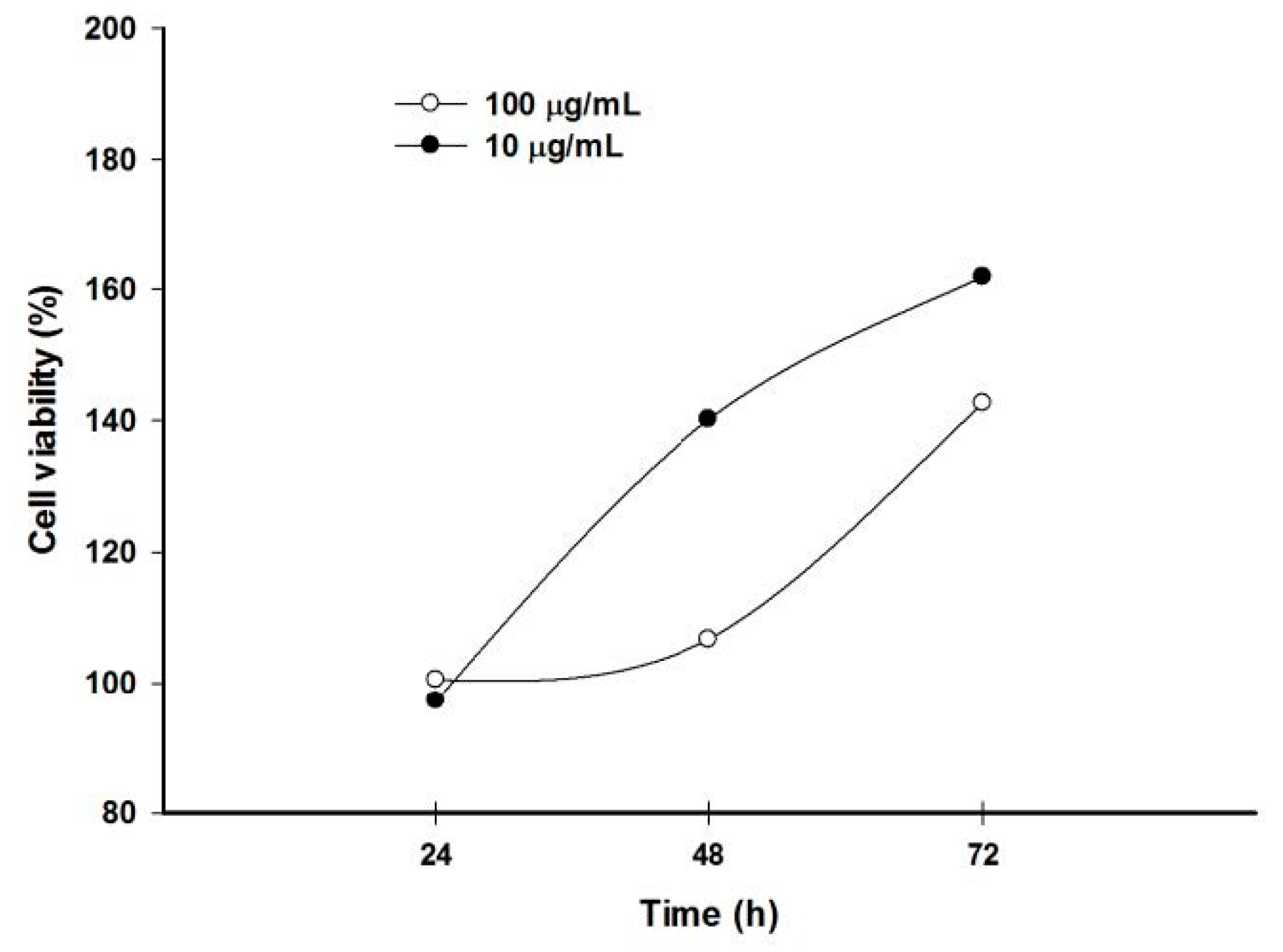
2.6. Pomegranate Polyphenols Inhibited HaCaT Cell Proliferation
2.7. Pomegranate Polyphenols Attenuated Heat-Killed P. acnes-Induced NO and PGE2 Production by RAW 264.7 Cells
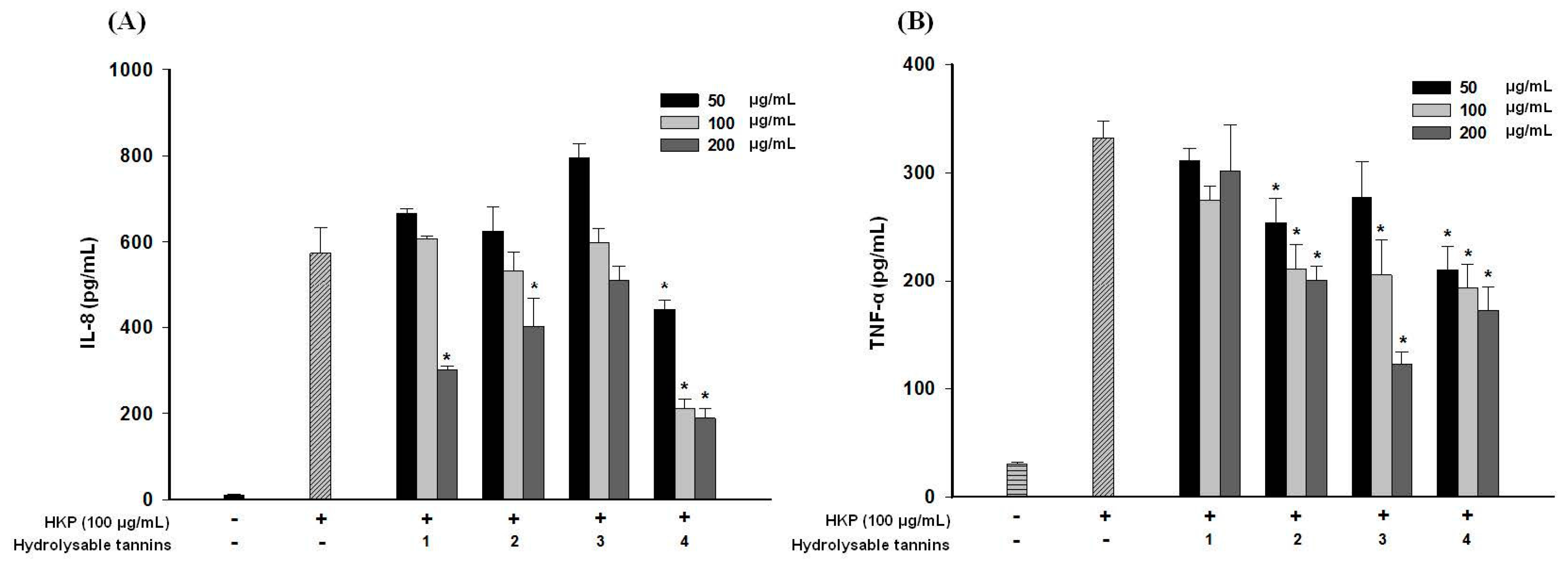
2.8. Pomegranate Polyphenols Attenuated Heat-Killed P. acnes-Induced IL-8 and TNF-α Production by THP-1 Cells
3. Materials and Methods
3.1. General
3.2. Preparation of Pomegranate Extract (PG-E)
3.3. P. acnes-Induced Ear Edema in Wistar Rats
3.4. Skin Irritation
3.5. Anti-Bacterial Activities against P. acnes and S. aureus
3.6. Scanning Electron Microscopy (SEM)
3.7. Anti-Lipase Activity
3.8. Anti-Proliferative Activity against Testosterone-Treated HaCaT Cells
3.9. Anti-Inflammatory Activity against Heat-Killed P. acnes-Treated RAW 264.7 Cells
3.10. Anti-Inflammatory Activity against Heat-Killed P. acnes-Treated THP-1 Cells
3.11. Statistical Analysis
4. Conclusions
Author Contributions
Conflicts of Interest
References
- James, W.D. Clinical practice: Acne. N. Engl. J. Med. 2005, 352, 1463–1472. [Google Scholar] [CrossRef] [PubMed]
- Pappas, A. Epidermal surface lipids. Dermatoendocrinology 2009, 1, 72–76. [Google Scholar] [CrossRef]
- Patel, M.; Bowe, W.P.; Heughebaert, C.; Shalita, A.R. The development of antimicrobial resistance due to the antibiotic treatment of acne vulgaris: A review. J. Drugs Dermatol. 2010, 9, 655–664. [Google Scholar] [PubMed]
- Youn, S.W. The role of facial sebum secretion in acne pathogenesis: Facts and controversies. Clin. Dermatol. 2010, 28, 8–11. [Google Scholar] [CrossRef] [PubMed]
- Olutunmbi, Y.; Paley, K.; English, J.C., 3rd. Adolescent female acne: Etiology and management. J. Pediatr. Adolesc. Gynecol. 2008, 21, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Leccia, M.T.; Auffret, N.; Poli, F.; Claudel, J.P.; Corvec, S.; Dreno, B. Topical acne treatments in Europe and the issue of antimicrobial resistance. J. Eur. Acad. Dermatol. Venereol. 2015, 29, 1485–1492. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.H.; Yoon, J.Y.; Park, S.Y.; Min, S.; Suh, D.H. Comparison of clinical and histological effects between lactobacillus-fermented Chamaecyparis obtusa and tea tree oil for the treatment of acne: An eight-week double-blind randomized controlled split-face study. Dermatology 2014, 229, 102–109. [Google Scholar] [CrossRef] [PubMed]
- Ha, H.; Lee, H.; Seo, C.S.; Lim, H.S.; Lee, J.K.; Lee, M.Y.; Shin, H. Artemisia capillaris inhibits atopic dermatitis-like skin lesions in Dermatophagoides farinae-sensitized Nc/Nga mice. BMC Complement. Altern. Med. 2014, 14, 100. [Google Scholar] [CrossRef] [PubMed]
- Kornhauser, A.; Wei, R.R.; Yamaguchi, Y.; Coelho, S.G.; Kaidbey, K.; Barton, C.; Takahashi, K.; Beer, J.Z.; Miller, S.A.; Hearing, V.J. The effects of topically applied glycolic acid and salicylic acid on ultraviolet radiation-induced erythema, DNA damage and sunburn cell formation in human skin. J. Dermatol. Sci. 2009, 55, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Plewig, G.; Holland, K.T.; Nenoff, P. Clinical and bacteriological evaluation of nadifloxacin 1% cream in patients with acne vulgaris: A double-blind, phase III comparison study versus erythromycin 2% cream. Eur. J. Dermatol. 2006, 16, 48–55. [Google Scholar] [PubMed]
- Sathishkumar, P.; Preethi, J.; Vijayan, R.; Mohd Yusoff, A.R.; Ameen, F.; Suresh, S.; Balagurunathan, R.; Palvannan, T. Anti-acne, anti-dandruff and anti-breast cancer efficacy of green synthesised silver nanoparticles using Coriandrum sativum leaf extract. J. Photochem. Photobiol. B 2016, 163, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Onumah, N. A novel anti-inflammatory in treatment of acne vulgaris: the pseudopterosins. J. Drugs Dermatol. 2013, 12, 1177–1179. [Google Scholar] [PubMed]
- Lee, C.J.; Chen, L.G.; Liang, W.L.; Wang, C.C. Anti-inflammatory effects of Punica granatum Linne in vitro and in vivo. Food Chem. 2010, 118, 315–322. [Google Scholar] [CrossRef]
- Lansky, E.P.; Newman, R.A. Punica granatum (pomegranate) and its potential for prevention and treatment of inflammation and cancer. J. Ethnopharmacol. 2007, 109, 177–206. [Google Scholar] [CrossRef] [PubMed]
- Reddy, M.K.; Gupta, S.K.; Jacob, M.R.; Khan, S.I.; Ferreira, D. Antioxidant, antimalarial and antimicrobial activities of tannin-rich fractions, ellagitannins and phenolic acids from Punica granatum L. Planta Med. 2007, 73, 461–467. [Google Scholar] [CrossRef] [PubMed]
- Menezes, S.M.; Cordeiro, L.N.; Viana, G.S. Punica granatum (pomegranate) extract is active against dental plaque. J. Herb. Pharmacother. 2006, 6, 79–92. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.J.; Chen, L.W.; Chen, L.G.; Chang, T.L.; Huang, C.W.; Huang, M.C.; Wang, C.C. Correlations of the components of tea tree oil with its antibacterial effects and skin irritation. J. Food Drug Anal. 2013, 21, 169–176. [Google Scholar] [CrossRef]
- Khan, N.; Afaq, F.; Kweon, M.H.; Kim, K.; Mukhtar, H. Oral consumption of pomegranate fruit extract inhibits growth and progression of primary lung tumors in mice. Cancer Res. 2007, 67, 3475–3482. [Google Scholar] [CrossRef] [PubMed]
- Abdollahzadeh, S.H.; Mashouf, R.; Mortazavi, H.; Moghaddam, M.; Roozbahani, N.; Vahedi, M. Antibacterial and antifungal activities of Punica granatum peel extracts against oral pathogens. J. Dent. (Tehran) 2011, 8, 1–6. [Google Scholar]
- Fawole, O.A.; Makunga, N.P.; Opara, U.L. Antibacterial, antioxidant and tyrosinase-inhibition activities of pomegranate fruit peel methanolic extract. BMC Complement. Altern. Med. 2012, 12, 200. [Google Scholar] [CrossRef] [PubMed]
- Al-Zoreky, N.S. Antimicrobial activity of pomegranate (Punica granatum L.) fruit peels. Int. J. Food Microbiol. 2009, 134, 244–248. [Google Scholar] [CrossRef] [PubMed]
- Azimi, H.; Fallah-Tafti, M.; Khakshur, A.A.; Abdollahi, M. A review of phytotherapy of acne vulgaris: Perspective of new pharmacological treatments. Fitoterapia 2012, 83, 1306–1317. [Google Scholar] [CrossRef] [PubMed]
- Rabie, E.; Serem, J.C.; Oberholzer, H.M.; Gaspar, A.R.; Bester, M.J. How methylglyoxal kills bacteria: An ultrastructural study. Ultrastruct. Pathol. 2016, 40, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Bakkiyaraj, D.; Nandhini, J.R.; Malathy, B.; Pandian, S.K. The anti-biofilm potential of pomegranate (Punica granatum L.) extract against human bacterial and fungal pathogens. Biofouling 2013, 29, 929–937. [Google Scholar] [CrossRef] [PubMed]
- Hadrich, F.; Cher, S.; Gargouri, Y.T.; Adel, S. Antioxidant and lipase inhibitory activities and essential oil composition of pomegranate peel extracts. J. Oleo Sci. 2014, 63, 515–525. [Google Scholar] [CrossRef] [PubMed]
- Baccarin, T.; Mitjans, M.; Ramos, D.; Lemos-Senna, E.; Vinardell, M.P. Photoprotection by Punica granatum seed oil nanoemulsion entrapping polyphenol-rich ethyl acetate fraction against UVB-induced DNA damage in human keratinocyte (HaCaT) cell line. J. Photochem. Photobiol. B 2015, 153, 127–136. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zaid, M.A.; Afaq, F.; Syed, D.N.; Dreher, M.; Mukhtar, H. Inhibition of UVB-mediated oxidative stress and markers of photoaging in immortalized HaCaT keratinocytes by pomegranate polyphenol extract POMx. Photochem. Photobiol. 2007, 83, 882–888. [Google Scholar] [CrossRef] [PubMed]
- Rottboell, L.; de Foenss, S.; Thomsen, K.; Christiansen, H.; Andersen, S.M.; Dam, T.N.; Rosada, C.; Stenderup, K. Exploring valrubicin’s effect on Propionibacterium acnes-induced skin inflammation in vitro and in vivo. Dermatol. Rep. 2015, 7, 6246. [Google Scholar] [CrossRef] [PubMed]
- Luo, H.; Lv, X.D.; Wang, G.E.; Li, Y.F.; Kurihara, H.; He, R.R. Anti-inflammatory effects of anthocyanins-rich extract from bilberry (Vaccinium myrtillus L.) on croton oil-induced ear edema and Propionibacterium acnes plus LPS-induced liver damage in mice. Int. J. Food Sci. Nutr. 2014, 65, 594–601. [Google Scholar] [CrossRef] [PubMed]
- Ado, M.A.; Abas, F.; Mohammed, A.S.; Ghazali, H.M. Anti- and pro-lipase activity of selected medicinal, herbal and aquatic plants, and structure elucidation of an anti-lipase compound. Molecules 2013, 18, 14651–14669. [Google Scholar] [CrossRef] [PubMed]
- Ko, E.; Choi, H.; Kim, B.; Kim, M.; Park, K.N.; Bae, I.H.; Sung, Y.K.; Lee, T.R.; Shin, D.W.; Bae, Y.S. Testosterone stimulates Duox1 activity through GPRC6A in skin keratinocytes. J. Biol. Chem. 2014, 289, 28835–28845. [Google Scholar] [CrossRef] [PubMed]
- Squaiella-Baptistão, C.C.; Teixeira, D.; Mussalem, J.S.; Ishimura, M.E.; Longo-Maugéri, I.M. Modulation of Th1/Th2 immune responses by killed Propionibacterium acnes and its soluble polysaccharide fraction in a type I hypersensitivity murine model: Induction of different activation status of antigen-presenting cells. J. Immunol. Res. 2015, 132083. [Google Scholar] [CrossRef]


| Pathogenesis of Acne Vulgaris | Epithelial Cell & Keratin Accumulation | Sebum Accumulation | Bacterial Growth | Skin Inflammation |
|---|---|---|---|---|
| In vitro experimental designs | Testosterone-induced HaCaT cell proliferation | Anti-lipase activity | Propionibacterium acnes Staphylococcus aureus | Heat-killed P. acnes-induced RAW 264.7 Heat-killed P. acnes-induced THP-1 |
| Effective hydrolysable tannins | Punicalagin (1) | Punicalin (2) | Strictinin A (3) | Granatin B (4) |
| PG-E Samples | Diameter of Inhibition Zone (mm) |
|---|---|
| PG-E (mg/disc) | P. acnes |
| 0.25 | 11.3 ± 0.6 |
| 0.5 | 15.1 ± 0.5 |
| 1.0 | 17.1 ± 1.4 |
| PG-E (mg/disc) | S. aureus |
| 2.5 | 12.6 ± 0.3 |
| 5.0 | 15.4 ± 0.9 |
| 10.0 | 15.9 ± 0.7 |
| Anti-Bacterial Activity | ||||
|---|---|---|---|---|
| MIC/MBC (µg/mL) | 1 | 2 | 3 | 4 |
| P. acnes | 6.25/12.5 | 6.25/12.5 | 12.5/25 | 100/- |
| S. aureus | 12.5/25 | 12.5/25 | 25/50 | 12.5/25 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, C.-J.; Chen, L.-G.; Liang, W.-L.; Wang, C.-C. Multiple Activities of Punica granatum Linne against Acne Vulgaris. Int. J. Mol. Sci. 2017, 18, 141. https://doi.org/10.3390/ijms18010141
Lee C-J, Chen L-G, Liang W-L, Wang C-C. Multiple Activities of Punica granatum Linne against Acne Vulgaris. International Journal of Molecular Sciences. 2017; 18(1):141. https://doi.org/10.3390/ijms18010141
Chicago/Turabian StyleLee, Chia-Jung, Lih-Geeng Chen, Wen-Li Liang, and Ching-Chiung Wang. 2017. "Multiple Activities of Punica granatum Linne against Acne Vulgaris" International Journal of Molecular Sciences 18, no. 1: 141. https://doi.org/10.3390/ijms18010141
APA StyleLee, C. -J., Chen, L. -G., Liang, W. -L., & Wang, C. -C. (2017). Multiple Activities of Punica granatum Linne against Acne Vulgaris. International Journal of Molecular Sciences, 18(1), 141. https://doi.org/10.3390/ijms18010141






