Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells
Abstract
:1. Introduction
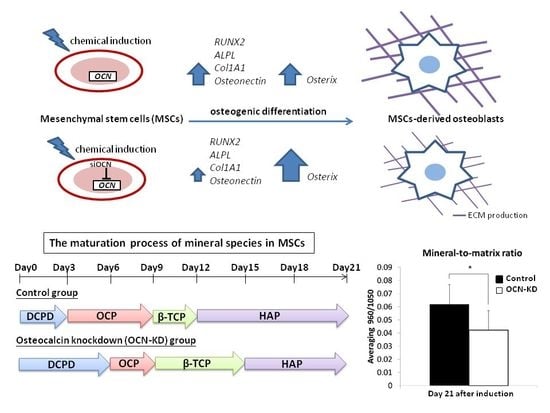
2. Results
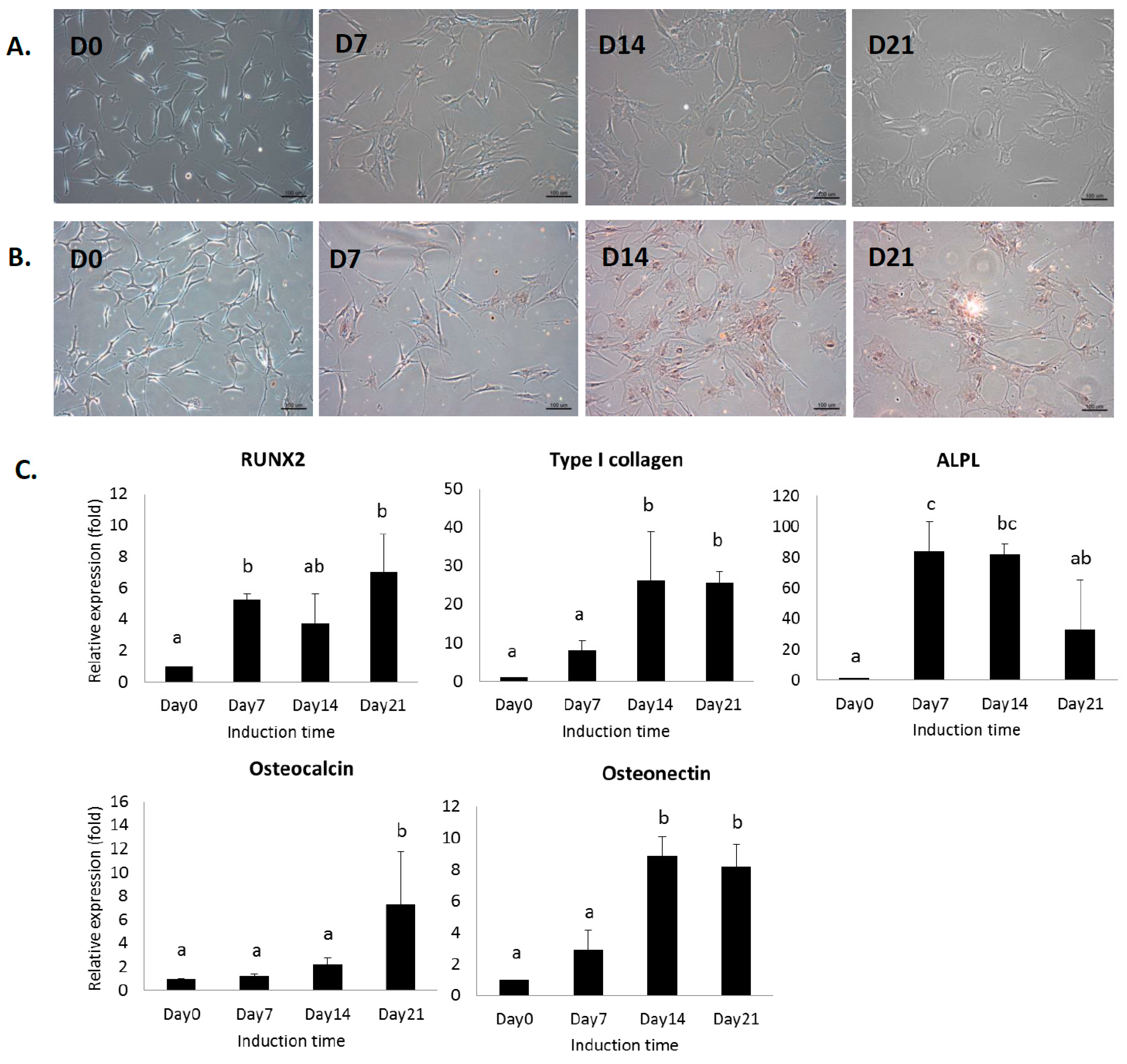
2.1. In Vitro Analysis of MSCs under Osteogenic Induction on Quartz Coverslips
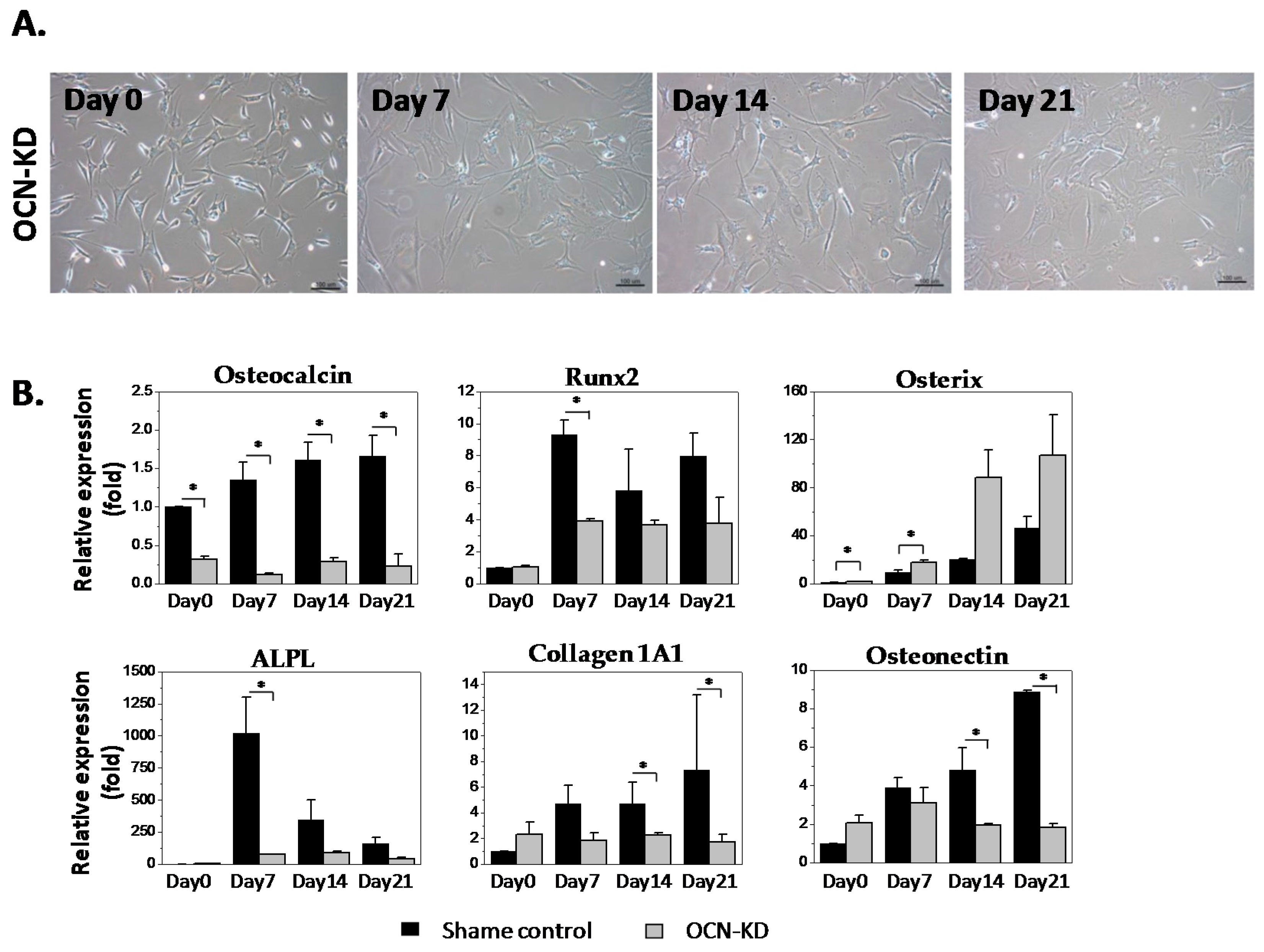
2.2. Gene Expression Analysis of MSCs after Osteocalcin Knockdown
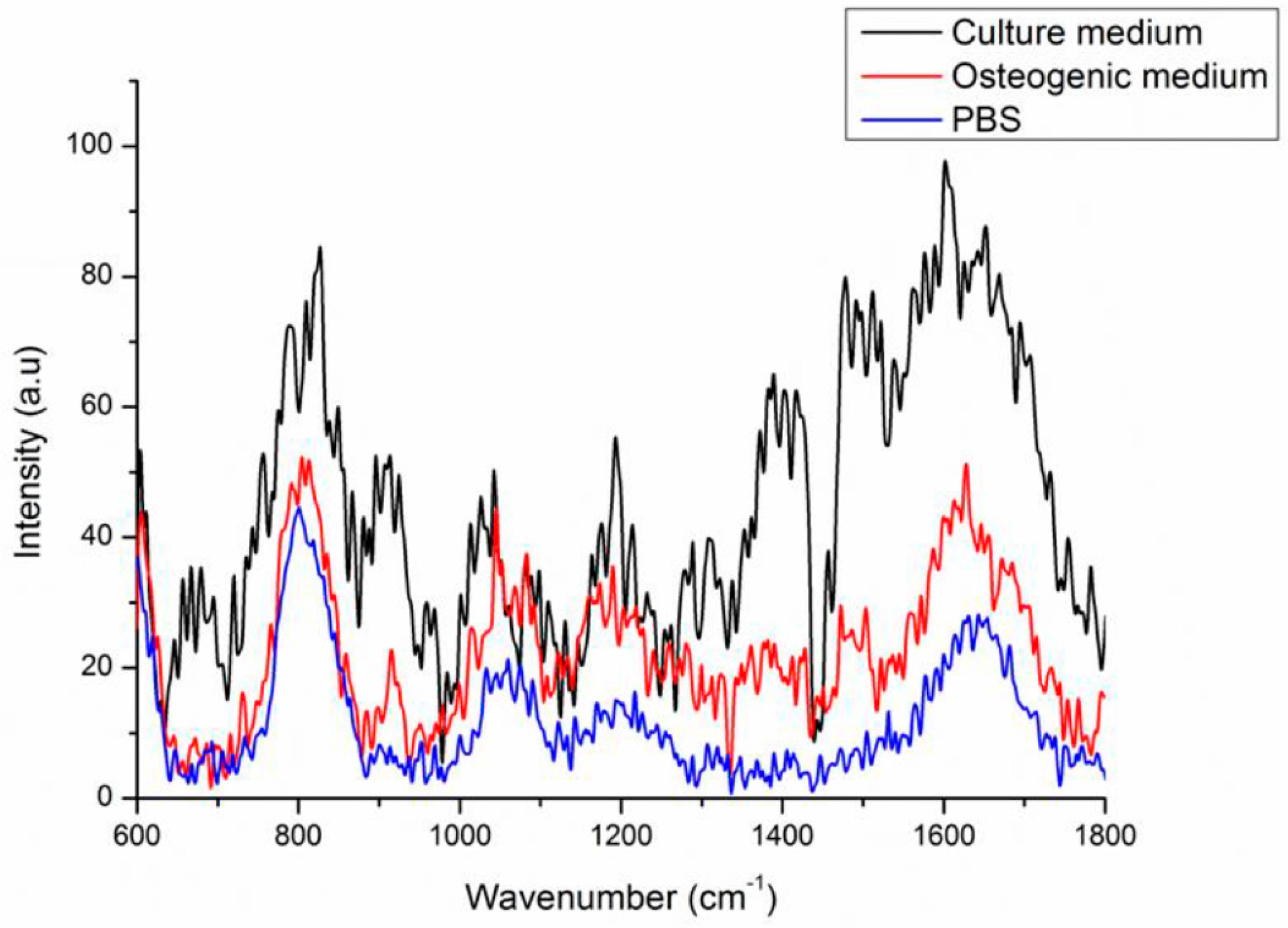
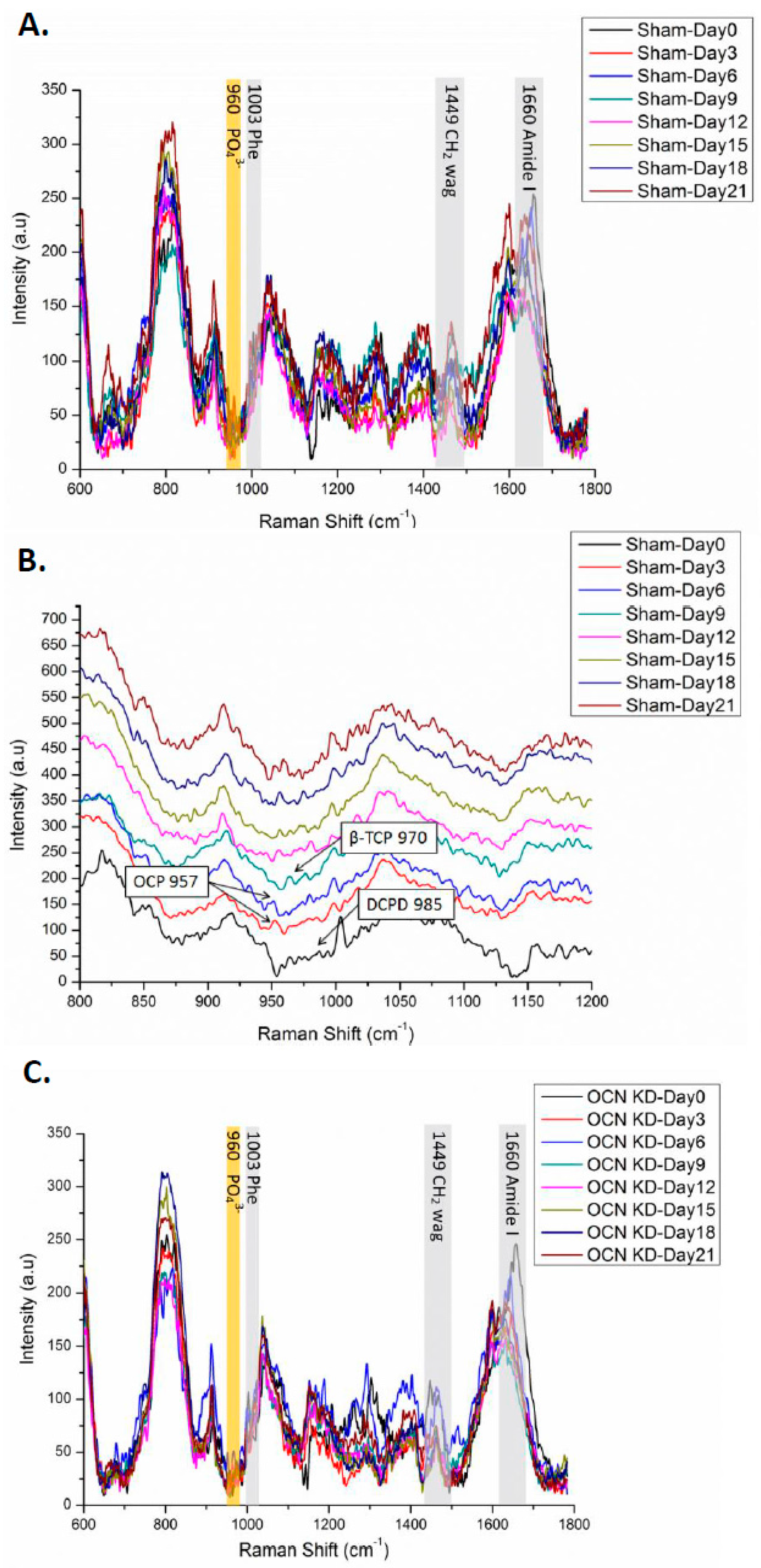
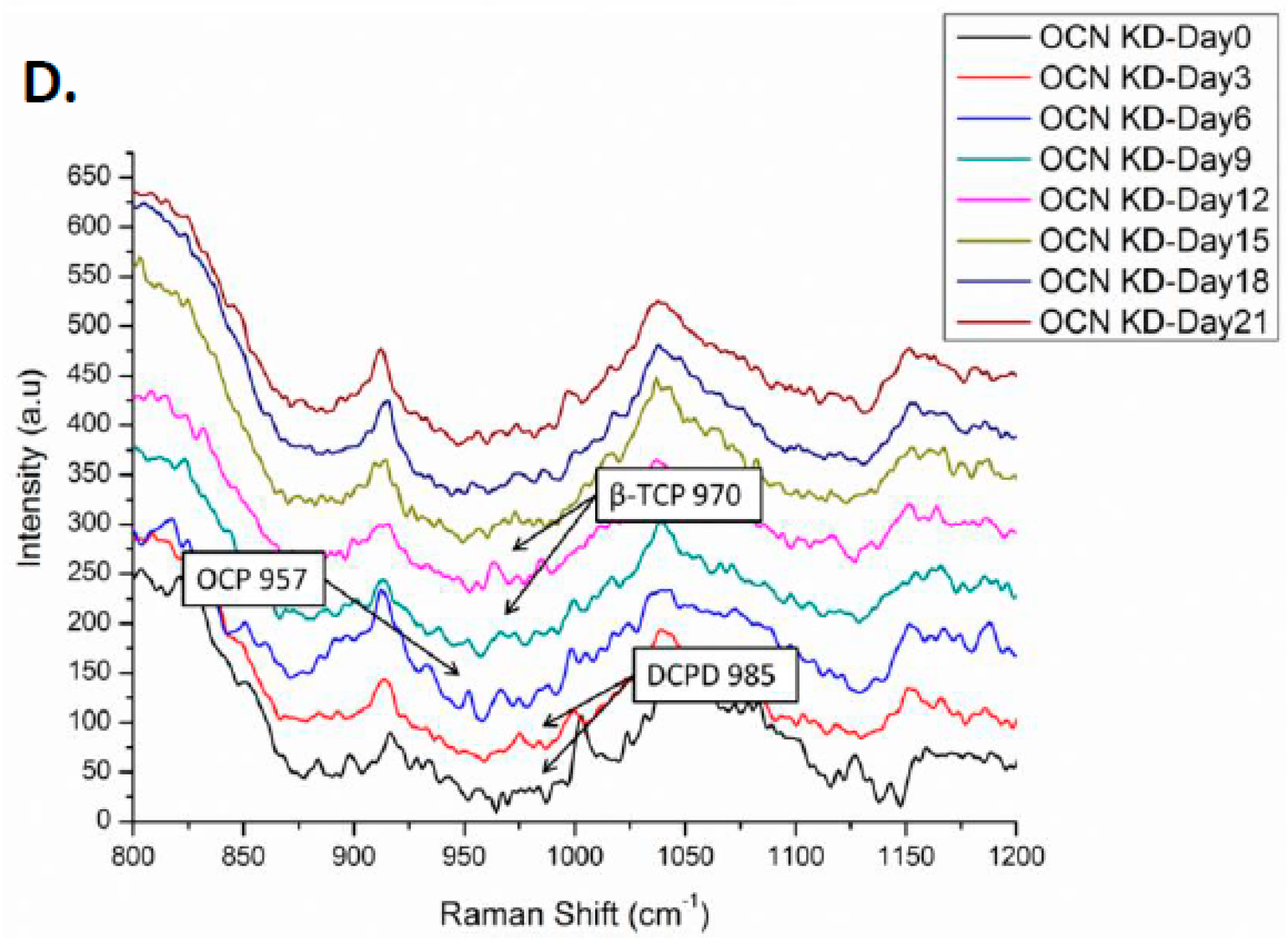
2.3. Background Signals of Raman
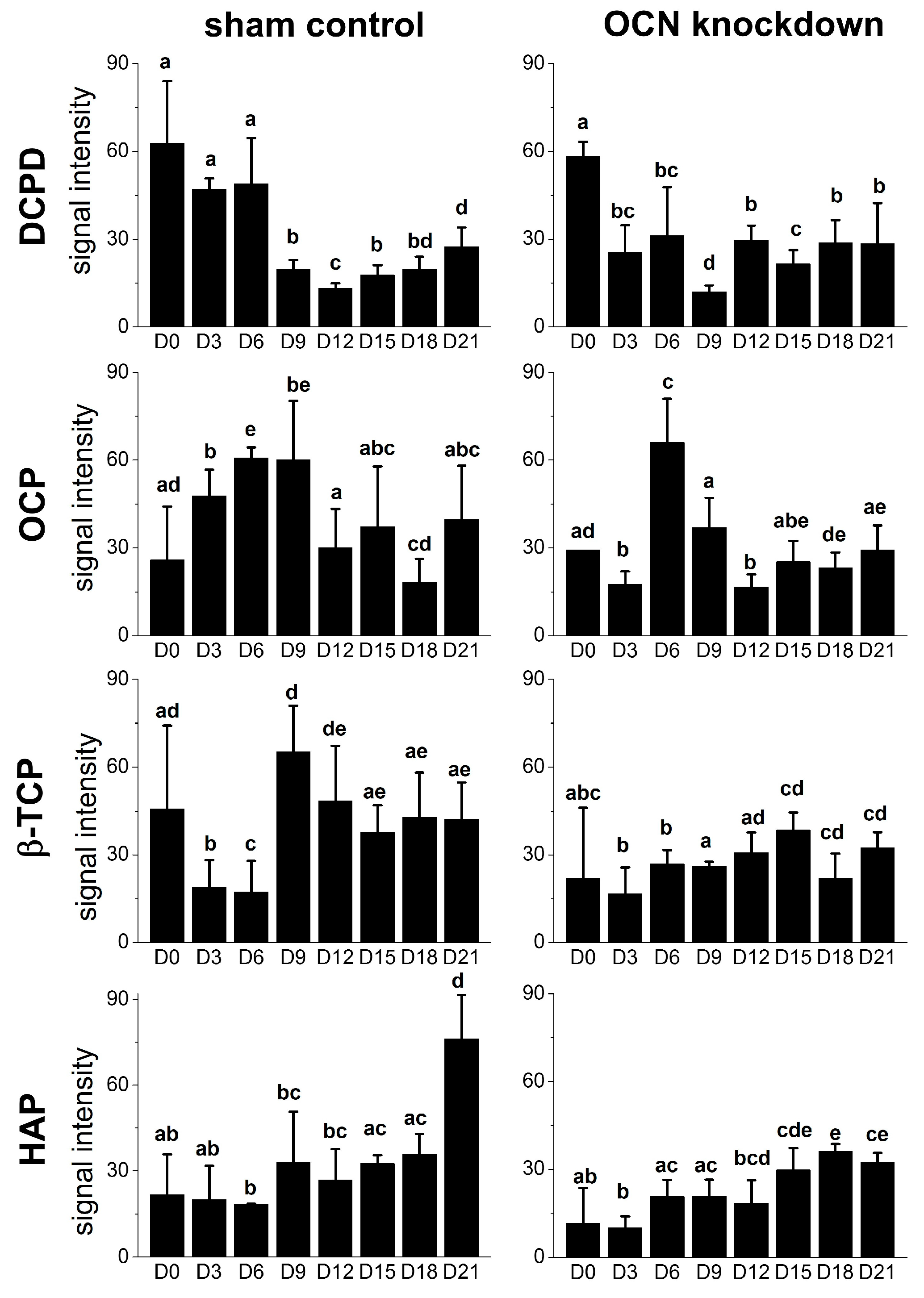
2.4. Raman Spectra of MSCs with Osteocalcin Knockdown
3. Discussion
4. Materials and Methods
4.1. Culture Maintenance and Osteogenic Differentiation of MSCs
4.2. siRNA Transfection
4.3. RNA Extraction and Real-Time Quantitative Polymerase Chain Reaction
4.4. Alizarin Red S Staining
4.5. Raman Spectroscopy
5. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| MSCs | mesenchymal stromal cells |
| OCN/BGLAP | osteocalcin/bone γ-carboxyglutamic acid-containing protein |
| HAP | hydroxyapatite |
| ACP | amorphous calcium phosphate |
| OCP | octacalcium phosphate |
| β-TCP | β-tricalcium phosphate |
| DCPD | dicalcium phosphate dehydrate |
| ON | osteonectin |
| ALPL | alkaline phosphatases |
| COL1A1 | type I collagen alpha 1 |
| OCN-KD | osteocalcin knockdown |
| PBS | phosphate-buffered saline |
| FBS | fetal bovine serum |
Appendix A
| Gene | Oligonucleotide Sequence | Probe |
|---|---|---|
| GAPDH | 5′-AGCCACATCGCTCAGACAC-3′/5′-GCCCAATACGACCAAATCC-3′ | 60 |
| RUNX2 | 5′-CACCATGTCAGCAAAACTTCTT-3′/5′-TCACGTCGCTCATTTTGC-3′ | 41 |
| Osteocalcin | 5′-GGCGCTACCTGTATCAATGG-3′/5′-TCAGCCAACTCGTCACAGTC-3′ | 1 |
| Osteonectin | 5′-GTGCAGAGGAAACCGAAGAG-3′/5′-TGTTTGCAGTGGTGGTTCTG-3′ | 77 |
| ALPL | 5′-AGAACCCCAAGGCTTCTTC-3′/5′-CTTGGCTTTTCCTTCATGGT-3′ | 31 |
| Type I collagen alpha 1 | 5′-CCCCTGGAAAGAATGGAGAT-3′/5′-AATCCTCGAGCACCCTGAG-3′ | 60 |
| Osterix/SP7 | 5′-GACTGCAGAGCAGGTTCCTC-3′/5′-TAACCTGATGGGGTCATGGT-3′ | 43 |
References
- Friedenstein, A.J.; Gorskaja, J.F.; Kulagina, N.N. Fibroblast precursors in normal and irradiated mouse hematopoietic organs. Exp. Hematol. 1976, 4, 267–274. [Google Scholar] [PubMed]
- Mouras, R.; Bagnaninchi, P.O.; Downes, A.R.; Elfick, A.P. Label-free assessment of adipose-derived stem cell differentiation using coherent anti-stokes Raman scattering and multiphoton microscopy. J. Biomed. Opt. 2012, 17, 116011. [Google Scholar] [CrossRef] [PubMed]
- Zuk, P.A.; Zhu, M.; Mizuno, H.; Huang, J.; Futrell, J.W.; Katz, A.J.; Benhaim, P.; Lorenz, H.P.; Hedrick, M.H. Multilineage cells from human adipose tissue: Implications for cell-based therapies. Tissue Eng. 2001, 7, 211–228. [Google Scholar] [CrossRef] [PubMed]
- Lee, O.K.; Kuo, T.K.; Chen, W.M.; Lee, K.D.; Hsieh, S.L.; Chen, T.H. Isolation of multipotent mesenchymal stem cells from umbilical cord blood. Blood 2004, 103, 1669–1675. [Google Scholar] [CrossRef] [PubMed]
- Da Silva Meirelles, L.; Caplan, A.I.; Nardi, N.B. In search of the in vivo identity of mesenchymal stem cells. Stem Cells 2008, 26, 2287–2299. [Google Scholar] [CrossRef] [PubMed]
- Crisan, M.; Yap, S.; Casteilla, L.; Chen, C.W.; Corselli, M.; Park, T.S.; Andriolo, G.; Sun, B.; Zheng, B.; Zhang, L.; et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell 2008, 3, 301–313. [Google Scholar] [CrossRef] [PubMed]
- Kuo, T.K.; Ho, J.H.; Lee, O.K. Mesenchymal stem cell therapy for nonmusculoskeletal diseases: Emerging applications. Cell Transplant. 2009, 18, 1013–1028. [Google Scholar] [CrossRef] [PubMed]
- Ghasroldasht, M.M.; Irfan-Maqsood, M.; Matin, M.M.; Bidkhori, H.R.; Naderi-Meshkin, H.; Moradi, A.; Bahrami, A.R. Mesenchymal stem cell based therapy for osteo-diseases. Cell Biol. Int. 2014, 38, 1081–1085. [Google Scholar] [CrossRef] [PubMed]
- Zomorodian, E.; Baghaban Eslaminejad, M. Mesenchymal stem cells as a potent cell source for bone regeneration. Stem Cells Int. 2012, 2012, 980353. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L. Mineral-matrix interactions in bone and cartilage. Clin. Orthop. Relat. Res. 1992, 281, 244–274. [Google Scholar] [CrossRef]
- Johnsson, M.S.; Nancollas, G.H. The role of brushite and octacalcium phosphate in apatite formation. Crit. Rev. Oral Biol. Med. 1992, 3, 61–82. [Google Scholar] [CrossRef] [PubMed]
- Stewart, S.; Shea, D.A.; Tarnowski, C.P.; Morris, M.D.; Wang, D.; Franceschi, R.; Lin, D.L.; Keller, E. Trends in early mineralization of murine calvarial osteoblastic cultures: A Raman microscopic study. J. Raman Spectrosc. 2002, 33, 536–543. [Google Scholar] [CrossRef]
- Hung, P.S.; Kuo, Y.C.; Chen, H.G.; Chiang, H.H.; Lee, O.K. Detection of osteogenic differentiation by differential mineralized matrix production in mesenchymal stromal cells by Raman spectroscopy. PLoS ONE 2013, 8, e65438. [Google Scholar] [CrossRef] [PubMed]
- Roach, H.I. Why does bone-matrix contain noncollagenous proteins—The possible roles of osteocalcin, osteonectin, osteopontin and bone sialoprotein in bone mineralization and resorption. Cell Biol. Int. 1994, 18, 617–628. [Google Scholar] [CrossRef] [PubMed]
- Boonrungsiman, S.; Gentleman, E.; Carzaniga, R.; Evans, N.D.; McComb, D.W.; Porter, A.E.; Stevens, M.M. The role of intracellular calcium phosphate in osteoblast-mediated bone apatite formation. Proc. Natl. Acad. Sci. USA 2012, 109, 14170–14175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, G. Function of the bone protein osteocalcin: Definitive evidence. Nutr. Rev. 1996, 54, 332–333. [Google Scholar] [CrossRef] [PubMed]
- Boskey, A.L.; Wians, F.H., Jr.; Hauschka, P.V. The effect of osteocalcin on in vitro lipid-induced hydroxyapatite formation and seeded hydroxyapatite growth. Calcif. Tissue Int. 1985, 37, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Knepper-Nicolai, B.; Reinstorf, A.; Hofinger, I.; Flade, K.; Wenz, R.; Pompe, W. Influence of osteocalcin and collagen I on the mechanical and biological properties of Biocement D. Biomol. Eng. 2002, 19, 227–231. [Google Scholar] [CrossRef]
- Rammelt, S.; Neumann, M.; Hanisch, U.; Reinstorf, A.; Pompe, W.; Zwipp, H.; Biewener, A. Osteocalcin enhances bone remodeling around hydroxyapatite/collagen composites. J. Biomed. Mater. Res. A 2005, 73, 284–294. [Google Scholar] [CrossRef] [PubMed]
- Ducy, P.; Desbois, C.; Boyce, B.; Pinero, G.; Story, B.; Dunstan, C.; Smith, E.; Bonadio, J.; Goldstein, S.; Gundberg, C.; et al. Increased bone formation in osteocalcin-deficient mice. Nature 1996, 382, 448–452. [Google Scholar] [CrossRef] [PubMed]
- Neve, A.; Corrado, A.; Cantatore, F.P. Osteocalcin: Skeletal and extra-skeletal effects. J. Cell. Physiol. 2013, 228, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Schulze, H.G.; Konorov, S.O.; Caron, N.J.; Piret, J.M.; Blades, M.W.; Turner, R.F.B. Assessing differentiation status of human embryonic stem cells noninvasively using Raman microspectroscopy. Anal. Chem. 2010, 82, 5020–5027. [Google Scholar] [CrossRef] [PubMed]
- Petry, R.; Schmitt, M.; Popp, J. Raman spectroscopy—A prospective tool in the life sciences. ChemPhysChem 2003, 4, 14–30. [Google Scholar] [CrossRef] [PubMed]
- Kendall, C.; Stone, N.; Shepherd, N.; Geboes, K.; Warren, B.; Bennett, R.; Barr, H. Raman spectroscopy, a potential tool for the objective identification and classification of neoplasia in Barrett’s oesophagus. J. Pathol. 2003, 200, 602–609. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, C.P.; Ignelzi, M.A.; Morris, M.D. Mineralization of developing mouse calvaria as revealed by Raman microspectroscopy. J. Bone Miner. Res. 2002, 17, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Puppels, G.J.; Demul, F.F.M.; Otto, C.; Greve, J.; Robertnicoud, M.; Arndtjovin, D.J.; Jovin, T.M. Studying single living cells and chromosomes by confocal Raman microspectroscopy. Nature 1990, 347, 301–303. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Verrier, S.; Haque, S.; Polak, J.M.; Hench, L.L. Spectroscopic study of human lung epithelial cells (A549) in culture: Living cells versus dead cells. Biopolymers 2003, 72, 230–240. [Google Scholar] [CrossRef] [PubMed]
- Notingher, I.; Bisson, I.; Bishop, A.E.; Randle, W.L.; Polak, J.M.; Hench, L.L. In situ spectral monitoring of mRNA translation in embryonic stem cells during differentiation in vitro. Anal. Chem. 2004, 76, 3185–3193. [Google Scholar] [CrossRef] [PubMed]
- McManus, L.L.; Burke, G.A.; McCafferty, M.M.; O’Hare, P.; Modreanu, M.; Boyd, A.R.; Meenan, B.J. Raman spectroscopic monitoring of the osteogenic differentiation of human mesenchymal stem cells. Analyst 2011, 136, 2471–2481. [Google Scholar] [CrossRef] [PubMed]
- Sauer, G.R.; Zunic, W.B.; Durig, J.R.; Wuthier, R.E. Fourier transform Raman spectroscopy of synthetic and biological calcium phosphates. Calcif. Tissue Int. 1994, 54, 414–420. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, A.; Ashton, L.; Yang, X.B.; Goodacre, R.; Smith, A.; Kirkham, J. Detection of early stage changes associated with adipogenesis using Raman spectroscopy under aseptic conditions. Cytom. A 2015, 87, 1012–1019. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, A.; Ashton, L.; Yang, X.B.; Goodacre, R.; Tomlinson, M.J.; Smith, A.; Kirkham, J. Aseptic Raman spectroscopy can detect changes associated with the culture of human dental pulp stromal cells in osteoinductive culture. Analyst 2015, 140, 7347–7354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanazawa, I. Osteocalcin as a hormone regulating glucose metabolism. World J. Diabetes 2015, 6, 1345–1354. [Google Scholar] [CrossRef] [PubMed]

© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsao, Y.-T.; Huang, Y.-J.; Wu, H.-H.; Liu, Y.-A.; Liu, Y.-S.; Lee, O.K. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. Int. J. Mol. Sci. 2017, 18, 159. https://doi.org/10.3390/ijms18010159
Tsao Y-T, Huang Y-J, Wu H-H, Liu Y-A, Liu Y-S, Lee OK. Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells. International Journal of Molecular Sciences. 2017; 18(1):159. https://doi.org/10.3390/ijms18010159
Chicago/Turabian StyleTsao, Yu-Tzu, Yi-Jeng Huang, Hao-Hsiang Wu, Yu-An Liu, Yi-Shiuan Liu, and Oscar K. Lee. 2017. "Osteocalcin Mediates Biomineralization during Osteogenic Maturation in Human Mesenchymal Stromal Cells" International Journal of Molecular Sciences 18, no. 1: 159. https://doi.org/10.3390/ijms18010159