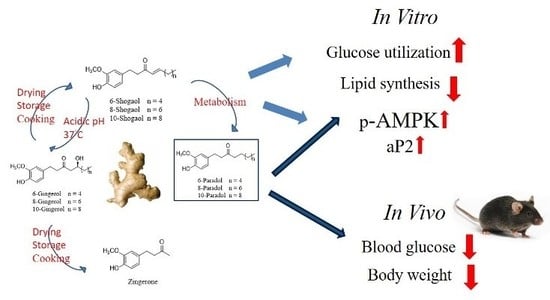
6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice
Abstract
:1. Introduction
2. Results
2.1. Ginger’s Non-Volatile Pungent Components Promote Glucose Utilization in 3T3-L1 Adipocytes
2.2. 6-Shogaol Promotes Glucose Utilization in 3T3-L1 Adipocytes and C2C12 Myotubes as Well as It Inhibits Lipid Synthesis in 3T3-L1 Adipocytes
2.3. The Effect of 6-, 8- or 10-Shogaol on Glucose Utilization and Lipid Synthesis
2.4. 6-Paradol Promotes Glucose Utilization in 3T3-L1 Adipocytes and C2C12 Myotubes as Well as It Inhibits Lipid Synthesis in 3T3-L1 Adipocytes
2.5. The Mechanisms of 6-Shogaol and 6-Paradol in Regulating Glucose Utilization and Lipid Accumulation in 3T3-L1 Adipocytes
2.6. The Anti-Hyperglycemic Effect of 6-Paradol in the High-Fat Diet Mouse Model
3. Discussion
4. Materials and Methods
4.1. Chemicals
4.2. Preparation of 6-Shogaol, 8-Shogaol, 10-Shogaol, 6-Paradol, 8-Paradol and 10-Paradol
4.3. Cell Culture and Differentiation
4.4. Medium Glucose Utilization Assay
4.5. Oil Red O Staining
4.6. Western Blot Analysis
4.7. Animal Model
4.8. Oral Glucose Tolerance Test
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Acknowledgments
Author Contributions
Conflicts of Interest
Abbreviations
| HFD | high-fat diet |
| GLUT4 | glucose transporter type 4 |
| LDL | low-density lipoprotein |
| VLDL | very-low-density lipoprotein |
| DMSO | dimethyl sulfoxide |
| EtOH | ethanol |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | fetal bovine serum |
| THF | tetrahydrofuran |
| tBuOK | potassium tertiary butoxide |
| Pd/C | palladium on carbon |
| AMPK | 5′ adenosine monophosphate-activated protein kinase |
| PPAR | peroxisome proliferator-activated receptor |
| aP2 | adipocyte fatty acid binding protein 4 |
| SREBP-1 | sterol regulatory element binding protein-1 |
| C/EBP-α | CCAAT enhancer binding protein |
| FAS | fatty acid synthase |
| OGTT | oral glucose tolerance test |
| BAT | brown adipose tissue |
References
- Butt, M.S.; Sultan, M.T. Ginger and its health claims: Molecular aspects. Crit. Rev. Food Sci. Nutr. 2011, 51, 383–393. [Google Scholar] [CrossRef] [PubMed]
- Shukla, Y.; Singh, M. Cancer preventive properties of ginger: A brief review. Food Chem. Toxicol. 2007, 45, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Al-Suhaimi, E.A.; Al-Riziza, N.A.; Al-Essa, R.A. Physiological and therapeutical roles of ginger and turmeric on endocrine functions. Am. J. Chin. Med. 2011, 39, 215–231. [Google Scholar] [CrossRef] [PubMed]
- White, B. Ginger: An overview. Am. Fam. Physician 2007, 75, 1689–1691. [Google Scholar] [PubMed]
- Jiang, H.; Xie, Z.; Koo, H.J.; McLaughlin, S.P.; Timmermann, B.N.; Gang, D.R. Metabolic profiling and phylogenetic analysis of medicinal Zingiber species: Tools for authentication of ginger (Zingiber officinale Rosc). Phytochemistry 2006, 67, 1673–1685. [Google Scholar] [CrossRef] [PubMed]
- Ali, B.H.; Blunden, G.; Tanira, M.O.; Nemmar, A. Some phytochemical, pharmacological and toxicological properties of ginger (Zingiber officinale Roscoe): A review of recent research. Food Chem. Toxicol. 2008, 46, 409–420. [Google Scholar] [CrossRef] [PubMed]
- Nicoll, R.; Henein, M.Y. Ginger (Zingiber officinale Roscoe): A hot remedy for cardiovascular disease? Int. J. Cardiol. 2009, 131, 408–409. [Google Scholar] [CrossRef] [PubMed]
- Altman, R.D.; Marcussen, K.C. Effects of a ginger extract on knee pain in patients with osteoarthritis. Arthritis Rheum. 2001, 44, 2531–2538. [Google Scholar] [CrossRef]
- Chrubasik, S.; Pittler, M.H.; Roufogalis, B.D. Zingiberis rhizoma: A comprehensive review on the ginger effect and efficacy profiles. Phytomedicine 2005, 12, 684–701. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.H.; Wu, P.Y.; Chen, K.M.; Fu, T.F.; Wang, H.M.; Chen, C.Y. Antiallergic potential on RBL-2H3 cells of some phenolic constituents of Zingiber officinale (ginger). J. Nat. Prod. 2009, 72, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Imam, K. Clinical features, diagnostic criteria and pathogenesis of diabetes mellitus. Adv. Exp. Med. Biol. 2012, 771, 340–355. [Google Scholar] [PubMed]
- Tahrani, A.A.; Barnett, A.H.; Bailey, C.J. Pharmacology and therapeutic implications of current drugs for type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2016, 12, 566–592. [Google Scholar] [CrossRef] [PubMed]
- Evans, J.L.; Balkan, B.; Chuang, E.; Rushakoff, R.J. Oral and injectable (non-insulin) pharmacological agents for type 2 diabetes. In Endotext; de Groot, L.J., Chrousos, G., Dungan, K., Feingold, K.R., Grossman, A., Hershmam, J.M., Koch, C., Korbonits, M., McLachlan, R., New, M., et al., Eds.; MDText.com, Inc.: South Dartmouth, MA, USA, 2000. [Google Scholar]
- Ginter, E.; Simko, V. Type 2 diabetes mellitus, pandemic in 21st century. Adv. Exp. Med. Biol. 2012, 771, 42–50. [Google Scholar] [PubMed]
- Andallu, B.; Radhika, B.; Suryakantham, V. Effect of aswagandha, ginger and mulberry on hyperglycemia and hyperlipidemia. Plant Foods Hum. Nutr. 2003, 58, 1–7. [Google Scholar] [CrossRef]
- Akhani, S.P.; Vishwakarma, S.L.; Goyal, R.K. Antidiabetic activity of Zingiber officinale in streptozotocin induced type I diabetic rats. J. Pharm. Pharmacol. 2004, 56, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Weidner, M.S.; Sigwart, K. The safety of a ginger extract in the rat. J. Ethnopharmacol. 2000, 73, 513–520. [Google Scholar] [CrossRef]
- Gong, F.; Fung, Y.S.; Liang, Y.Z. Determination of volatile components in ginger using gas chromatography-mass spectrometry with resolution improved by data processing techniques. J. Agric. Food Chem. 2004, 52, 6378–6383. [Google Scholar] [CrossRef] [PubMed]
- Sekiya, K.; Ohtani, A.; Kusano, S. Enhancement of insulin sensitivity in adipocytes by ginger. BioFactors 2004, 22, 153–156. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tran, V.H.; Duke, C.C.; Roufogalis, B.D. Gingerols of Zingiber officinale enhance glucose uptake by increasing cell surface GLUT4 in cultured L6 myotubes. Planta Med. 2012, 78, 1549–1555. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.O.; Kim, N.; Lee, H.J.; Moon, J.W.; Lee, S.K.; Kim, S.J.; Kim, J.K.; Park, S.H.; Kim, H.S. [6]-Gingerol affects glucose metabolism by dual regulation via the AMPKα2-mediated AS160-Rab5 pathway and AMPK-mediated insulin sensitizing effects. J. Cell. Biochem. 2015, 116, 1401–1410. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Tran, V.H.; Koolaji, N.; Duke, C.; Roufogalis, B.D. (S)-[6]-Gingerol enhances glucose uptake in L6 myotubes by activation of AMPK in response to [Ca2+]i. J. Pharm. Pharm. Sci. 2013, 16, 304–312. [Google Scholar] [CrossRef] [PubMed]
- Son, M.J.; Miura, Y.; Yagasaki, K. Mechanisms for antidiabetic effect of gingerol in cultured cells and obese diabetic model mice. Cytotechnology 2015, 67, 641–652. [Google Scholar] [CrossRef] [PubMed]
- Govindarajan, V.S. Ginger-chemistry, technology, and quality evaluation: Part 1. Crit. Rev. Food Sci. Nutr. 1982, 17, 1–96. [Google Scholar] [CrossRef] [PubMed]
- Wohlmuth, H.; Leach, D.N.; Smith, M.K.; Myers, S.P. Gingerol content of diploid and tetraploid clones of ginger (Zingiber officinale Roscoe). J. Agric. Food Chem. 2005, 53, 5772–5778. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Lv, L.; Soroka, D.; Warin, R.F.; Parks, T.A.; Hu, Y.; Zhu, Y.; Chen, X.; Sang, S. Metabolism of [6]-shogaol in mice and in cancer cells. Drug Metab. Dispos. 2012, 40, 742–753. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.T.; Lee, M.S.; Kim, H.J.; Sung, M.J.; Kim, H.Y.; Kim, M.S.; Kwon, D.Y. Antiobesity effect of ginsenoside Rg3 involves the AMPK and PPAR-γ signal pathways. Phytother. Res. 2008, 23, 262–266. [Google Scholar] [CrossRef] [PubMed]
- Tuncman, G.; Erbay, E.; Hom, X.; de Vivo, I.; Campos, H.; Rimm, E.B.; Hotamisligil, G.S. A genetic variant at the fatty acid-binding protein aP2 locus reduces the risk for hypertriglyceridemia, type 2 diabetes, and cardiovascular disease. Proc. Natl. Acad. Sci. USA 2006, 103, 6970–6975. [Google Scholar] [CrossRef] [PubMed]
- Valverde, A.M.; Benito, M.; Lorenzo, M. The brown adipose cell: A model for understanding the molecular mechanisms of insulin resistance. Acta Physiol. Scand. 2005, 183, 59–73. [Google Scholar] [CrossRef] [PubMed]
- Han, J.H.; Zhou, W.; Li, W.; Tuan, P.Q.; Khoi, N.M.; Thuong, P.T.; Na, M.; Myung, C.S. Pentacyclic triterpenoids from Astilbe rivularis that enhance glucose uptake via the activation of Akt and Erk1/2 in C2C12 myotubes. J. Nat. Prod. 2015, 78, 1005–1014. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.J.; Tsai, Y.H.; Liao, M.C.; Du, Y.C.; Lien, P.J.; Sun, C.C.; Chang, F.R.; Wu, Y.C. Anti-diabetic properties of non-polar Toona sinensis Roem extract prepared by supercritical-CO2 fluid. Food Chem. Toxicol. 2012, 50, 779–789. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.T.; Hsieh, T.J.; El-Shazly, M.; Chuang, D.W.; Tsai, Y.H.; Yen, C.T.; Wu, S.F.; Wu, Y.C.; Chang, F.R. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg. Med. Chem. Lett. 2012, 22, 3912–3915. [Google Scholar] [CrossRef] [PubMed]
- Haratake, A.; Watase, D.; Setoguchi, S.; Terada, K.; Matsunaga, K.; Takata, J. Relationship between the acyl chain length of paradol analogues and their antiobesity activity following oral ingestion. J. Agric. Food Chem. 2014, 62, 6166–6174. [Google Scholar] [CrossRef] [PubMed]
- Nie, H.; Meng, L.Z.; Zhang, H.; Zhang, J.Y.; Yin, Z.; Huang, X.S. Analysis of anti-platelet aggregation components of Rhizoma Zingiberis using chicken thrombocyte extract and high performance liquid chromatography. Chin. Med. J. (Engl.) 2008, 121, 1226–1229. [Google Scholar] [PubMed]
- Suekawa, M.; Ishige, A.; Yuasa, K.; Sudo, K.; Aburada, M.; Hosoya, E.J. Pharmacological studies on ginger. I. Pharmacological actions of pungent constituents, (6)-gingerol and (6)-shogaol. J. Pharmacobio-dynam. 1984, 7, 836–848. [Google Scholar] [CrossRef]
- Pan, M.H.; Hsieh, M.C.; Kuo, J.M.; Lai, C.S.; Wu, H.; Sang, S.; Ho, C.T. 6-Shogaol induces apoptosis in human colorectal carcinoma cells via ROS production, caspase activation, and GADD 153 expression. Mol. Nutr. Food Res. 2008, 52, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Isa, Y.; Miyakawa, Y.; Yanagisawa, M.; Goto, T.; Kang, M.S.; Kawada, T.; Morimitsu, Y.; Kubota, K.; Tsuda, T. 6-Shogaol and 6-gingerol, the pungent of ginger, inhibit TNF-α mediated downregulation of adiponectin expression via different mechanisms in 3T3-L1 adipocytes. Biochem. Biophys. Res. Commun. 2008, 373, 429–434. [Google Scholar] [CrossRef] [PubMed]
- Kubra, I.R.; Rao, L.J. An impression on current developments in the technology, chemistry, and biological activities of ginger (Zingiber officinale Roscoe). Crit. Rev. Food Sci. Nutr. 2012, 52, 651–688. [Google Scholar] [CrossRef] [PubMed]
- Hu, F.B. Sedentary lifestyle and risk of obesity and type 2 diabetes. Lipids 2003, 38, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Shah, P.; Mudaliar, S. Pioglitazone: Side effect and safety profile. Expert Opin. Drug Saf. 2010, 9, 347–354. [Google Scholar] [CrossRef] [PubMed]
- Shih, H.C.; Chern, C.Y.; Kuo, P.C.; Wu, Y.C.; Chan, Y.Y.; Liao, Y.R.; Teng, C.M.; Wu, T.S. Synthesis of analogues of gingerol and shogaol, the active pungent principles from the rhizomes of Zingiber officinale and evaluation of their anti-platelet aggregation effects. Int. J. Mol. Sci. 2004, 15, 3926–3951. [Google Scholar] [CrossRef] [PubMed]
- Dagon, Y.; Avraham, Y.; Berry, E.M. AMPK activation regulates apoptosis, adipogenesis, and lipolysis by eIF2α in adipocytes. Biochem. Biophys. Res. Commun. 2006, 340, 43–47. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.B.; Zhou, G.; Li, C. AMPK: An emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 2009, 9, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.D.; Plutzky, J. Peroxisome proliferator-activated receptors as transcriptional nodal points and therapeutic targets. Circulation 2007, 115, 518–533. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Qi, Y.; Huang, T.H.; Yamahara, J.; Roufogalis, B.D. Pomegranate flower: A unique traditional antidiabetic medicine with dual PPAR-α/-γ activator properties. Diabetes Obes. Metab. 2008, 10, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Bhattarai, S.; Tran, V.H.; Duke, C.C. The stability of gingerol and shogaol in aqueous solutions. J. Pharm. Sci. 2001, 90, 1658–1664. [Google Scholar] [CrossRef] [PubMed]
- Berti, L.; Kellerer, M.; Capp, E.; Haring, H.U. Leptin stimulates glucose transport and glycogen synthesis in C2C12 myotubes: Evidence for a PI3-kinase mediated effect. Diabetologia 1997, 40, 606–609. [Google Scholar] [CrossRef] [PubMed]
- Ishiyama, M.; Shiga, M.; Sakamoto, K.; Mizoguchi, M.; He, P.A. New sulfonated tetrazolium salt that produces a highly watersoluble formazan dye. Chem. Pharm. Bull. 1993, 41, 1118–1122. [Google Scholar] [CrossRef]
- Srinivasan, K.; Ramarao, P. Animal models in type 2 diabetes research: An overview. Indian J. Med. Res. 2007, 125, 451–472. [Google Scholar] [PubMed]
- Zhou, M.; Xu, H.; Pan, L.; Wen, J.; Liao, W.; Chen, K. Rosiglitazone promotes atherosclerotic plaque stability in fat-fed ApoE-knockout mice. Eur. J. Pharmacol. 2008, 590, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Fang, Y.P.; Chuang, C.H.; Wu, P.C.; Huang, Y.B.; Tzeng, C.C.; Chen, Y.L.; Liu, Y.T.; Tsai, Y.H.; Tsai, M.J. Amsacrine analog-loaded solid lipid nanoparticle to resolve insolubility for injection delivery: Characterization and pharmacokinetics. Drug Des. Dev. Ther. 2016, 10, 1019–1028. [Google Scholar] [CrossRef] [PubMed]






| Parameters | Control | HFD | HFD + 6-Paradol (6.75 mg/kg/day) | HFD + 6-Paradol (33.75 mg/kg/day) | HFD + Pioglitazone (6.75 mg/kg/day) |
|---|---|---|---|---|---|
| n = 9 | n = 8 | n = 7 | n = 6 | n = 6 | |
| Fasting glucose (mg/dL) | 176.2 ± 7.5 c | 242.1 ± 13.5 | 201.93 ± 17.8 | 151 ± 12.3 b | 210 ± 27.8 |
| Triglycerol (mg/dL) | 49.2 ± 4.2 | 59.4 ± 4.9 | 56.3 ± 4.4 | 51.4 ± 8.0 | 64.3 ± 8.3 |
| T-CHO (mg/dL) | 65.9 ± 3.1 a | 148.1 ± 8.6 | 111 ± 3.4 a | 104.4 ± 7.8 a | 119.3 ± 5.2 b |
| ALT (U/L) | 36.5 ± 2.7 b | 103.1 ± 23.3 | 25.2 ± 4.8 a | 38.6 ± 8.2 b | 42.45 ± 8.8 c |
| Creatinine (mg/dL) | 0.17 ± 0.02 | 0.18 ± 0.01 | 0.20 ± 0.01 | 0.27 ± 0.03 b | 0.21 ± 0.02 |
© 2017 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wei, C.-K.; Tsai, Y.-H.; Korinek, M.; Hung, P.-H.; El-Shazly, M.; Cheng, Y.-B.; Wu, Y.-C.; Hsieh, T.-J.; Chang, F.-R. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. Int. J. Mol. Sci. 2017, 18, 168. https://doi.org/10.3390/ijms18010168
Wei C-K, Tsai Y-H, Korinek M, Hung P-H, El-Shazly M, Cheng Y-B, Wu Y-C, Hsieh T-J, Chang F-R. 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. International Journal of Molecular Sciences. 2017; 18(1):168. https://doi.org/10.3390/ijms18010168
Chicago/Turabian StyleWei, Chien-Kei, Yi-Hong Tsai, Michal Korinek, Pei-Hsuan Hung, Mohamed El-Shazly, Yuan-Bin Cheng, Yang-Chang Wu, Tusty-Jiuan Hsieh, and Fang-Rong Chang. 2017. "6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice" International Journal of Molecular Sciences 18, no. 1: 168. https://doi.org/10.3390/ijms18010168
APA StyleWei, C. -K., Tsai, Y. -H., Korinek, M., Hung, P. -H., El-Shazly, M., Cheng, Y. -B., Wu, Y. -C., Hsieh, T. -J., & Chang, F. -R. (2017). 6-Paradol and 6-Shogaol, the Pungent Compounds of Ginger, Promote Glucose Utilization in Adipocytes and Myotubes, and 6-Paradol Reduces Blood Glucose in High-Fat Diet-Fed Mice. International Journal of Molecular Sciences, 18(1), 168. https://doi.org/10.3390/ijms18010168